Do you know your PSMA-tracer? Variability in the biodistribution of different PSMA ligands and its potential impact on defining PSMA-positivity prior to PSMA-targeted therapy
- PMID: 39792324
- PMCID: PMC11723865
- DOI: 10.1186/s13550-024-01190-7
Do you know your PSMA-tracer? Variability in the biodistribution of different PSMA ligands and its potential impact on defining PSMA-positivity prior to PSMA-targeted therapy
Abstract
Background: In clinical practice, several radiopharmaceuticals are used for PSMA-PET imaging, each with distinct biodistribution patterns. This may impact treatment decisions and outcomes, as eligibility for PSMA-directed radioligand therapy is usually assessed by comparing tumoral uptake to normal liver uptake as a reference. In this study, we aimed to compare tracer uptake intraindividually in various reference regions including liver, parotid gland and spleen as well as the respective tumor-to-background ratios (TBR) of different 18F-labeled PSMA ligands to today's standard radiopharmaceutical 68Ga-PSMA-11 in a series of patients with biochemical recurrence of prostate cancer who underwent a dual PSMA-PET examination as part of an individualized diagnostic approach.
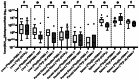
Results: Differences in background activity among different PSMA-PET tracers lead to variations in tumor-to-background ratios (TBR). In [18F]F-DCFPyL-PET, TBR with the liver as the reference organ (TBRliver) was comparable to [68Ga]Ga-PSMA-11-PET, while [18F]F-PSMA-1007-PET and [18F]F-JK-PSMA-7-PET showed significantly lower values. Using the parotid gland as the reference (TBRparotidgland), [18F]F-DCFPyL-PET exhibited significantly higher values, whereas [18F]F-PSMA-1007-PET and [18F]F-JK-PSMA-7-PET were comparable. For the spleen (TBRspleen), [18F]F-JK-PSMA-7-PET was comparable, but [18F]F-DCFPyL-PET and [18F]F-PSMA-1007-PET showed significantly higher and lower values, respectively. An additional Bland-Altman analyses revealed low bias for [18F]F-DCFPyL-PET in TBRparotidgland, whereas significant differences in TBRliver and TBRspleen for the other tracers resulted in higher bias.
Conclusion: Different PSMA-PET tracers exhibit distinct biodistribution patterns, leading to variations in tumor-to-background ratios (TBR) in reference organs such as the liver, parotid gland, and spleen. Patient selection for PSMA-directed radioligand therapy is currently based on a semiquantitative approach using the liver as a reference region in [68Ga]Ga-PSMA-11-PET. Thus, the use of alternative [18F]-labeled tracers may result in under- or overestimation of a patient's suitability for therapy. This highlights the importance of a comprehensive understanding of the differences in tracer-specific uptake behavior for accurate decisions regarding PSMA-expression levels. However, as the patient cohort in this study is at earlier disease stages, the generalizability of these findings to later-stage patients remains unclear and requires further investigation.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The Ethics Commission of the Faculty of Medicine of Cologne University has waived the need for approval. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. All enrolled patients gave written informed consent to participate in this study. Consent for publication: All enrolled patients gave written informed consent for the publication of this study and its accompanying images. Competing interests: A.D. discloses research support from Siemens Healthineers, Life Molecular Imaging, GE Healthcare, AVID Radiopharmaceuticals, SOFIE, Eisai and Novartis/AAA; Speaker Honorary and/or Advisory Boards fees from Siemens Healthineers, Sanofi, GE Healthcare, Biogen, Novo Nordisk, Invicro, Novartis/AAA and Bayer Vital; Stock from Siemens Healthineers, Lantheus Holding; a patent granted for 18 F-PSMA-JK-7 (PSMA-PET imaging tracer). J.H. discloses Advisory Boards fees from Novartis Pharma GmbH.
Figures

Similar articles
-
Intra-individual comparison of 68Ga-PSMA-11 and 18F-DCFPyL normal-organ biodistribution.Cancer Imaging. 2019 May 15;19(1):23. doi: 10.1186/s40644-019-0211-y. Cancer Imaging. 2019. PMID: 31092293 Free PMC article.
-
More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT.Hell J Nucl Med. 2019 Jan-Apr;22(1):6-9. doi: 10.1967/s002449910952. Epub 2019 Mar 7. Hell J Nucl Med. 2019. PMID: 30843003
-
Threshold for defining PSMA-positivity prior to 177Lu-PSMA therapy: a comparison of [68Ga]Ga-PSMA-11 and [18F]F-DCFPyL in metastatic prostate cancer.EJNMMI Res. 2023 Sep 20;13(1):83. doi: 10.1186/s13550-023-01033-x. EJNMMI Res. 2023. PMID: 37731097 Free PMC article.
-
PSA-Stratified Performance of 18F- and 68Ga-PSMA PET in Patients with Biochemical Recurrence of Prostate Cancer.J Nucl Med. 2017 Jun;58(6):947-952. doi: 10.2967/jnumed.116.185538. Epub 2016 Dec 1. J Nucl Med. 2017. PMID: 27908968 Clinical Trial.
-
Comparison of [(18)F]DCFPyL and [ (68)Ga]Ga-PSMA-HBED-CC for PSMA-PET Imaging in Patients with Relapsed Prostate Cancer.Mol Imaging Biol. 2015 Aug;17(4):575-84. doi: 10.1007/s11307-015-0866-0. Mol Imaging Biol. 2015. PMID: 26013479 Free PMC article.
References
-
- Parent EE, Savir-Baruch B, Gayed IW, Almaguel F, Chin BB, Pantel AR, et al. (177)Lu-PSMA Therapy. J Nucl Med Technol. 2022;50:205–12. 10.2967/jnmt.122.263814. - PubMed
-
- Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397:797–804. 10.1016/S0140-6736(21)00237-3. - PubMed
-
- Alberts IL, Seifert R, Rahbar K, Afshar-Oromieh A. Prostate Cancer Theranostics: from target description to imaging. PET Clin. 2021;16:383–90. 10.1016/j.cpet.2021.03.003. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

