Surprising features of nuclear receptor interaction networks revealed by live-cell single-molecule imaging
- PMID: 39792435
- PMCID: PMC11723585
- DOI: 10.7554/eLife.92979
Surprising features of nuclear receptor interaction networks revealed by live-cell single-molecule imaging
Abstract
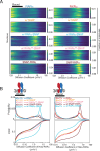
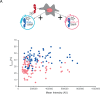
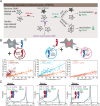
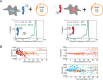
Type II nuclear receptors (T2NRs) require heterodimerization with a common partner, the retinoid X receptor (RXR), to bind cognate DNA recognition sites in chromatin. Based on previous biochemical and overexpression studies, binding of T2NRs to chromatin is proposed to be regulated by competition for a limiting pool of the core RXR subunit. However, this mechanism has not yet been tested for endogenous proteins in live cells. Using single-molecule tracking (SMT) and proximity-assisted photoactivation (PAPA), we monitored interactions between endogenously tagged RXR and retinoic acid receptor (RAR) in live cells. Unexpectedly, we find that higher expression of RAR, but not RXR, increases heterodimerization and chromatin binding in U2OS cells. This surprising finding indicates the limiting factor is not RXR but likely its cadre of obligate dimer binding partners. SMT and PAPA thus provide a direct way to probe which components are functionally limiting within a complex TF interaction network providing new insights into mechanisms of gene regulation in vivo with implications for drug development targeting nuclear receptors.
Keywords: chromosomes; gene expression; gene regulatory networks; human; molecular biophysics; nuclear receptors; protein-protein interactions; single-molecule tracking; structural biology; transcription factors.
© 2023, Dahal et al.
Conflict of interest statement
LD, GD, RT No competing interests declared, TG is an inventor of pending patent application (PCT/US2021/062616) related to the use of PAPA as a molecular proximity sensor, AH is currently an employee of Eikon Therapeutics, XD is a co-founder of Eikon Therapeutics, Inc and an inventor on a pending patent application (PCT/US2021/062616) related to the use of PAPA as a molecular proximity sensor
Figures





Update of
-
Surprising Features of Nuclear Receptor Interaction Networks Revealed by Live Cell Single Molecule Imaging.bioRxiv [Preprint]. 2024 Oct 30:2023.09.16.558083. doi: 10.1101/2023.09.16.558083. bioRxiv. 2024. Update in: Elife. 2025 Jan 10;12:RP92979. doi: 10.7554/eLife.92979. PMID: 37745337 Free PMC article. Updated. Preprint.
References
-
- Bouchard C, Dittrich O, Kiermaier A, Dohmann K, Menkel A, Eilers M, Lüscher B. Regulation of cyclin D2 gene expression by the myc/max/mad network: myc-dependent TRRAP recruitment and histone acetylation at the cyclin D2 promoter. Genes & Development. 2001;15:2042–2047. doi: 10.1101/gad.907901. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

