The adiponectin-PPARγ axis in hepatic stellate cells regulates liver fibrosis
- PMID: 39792554
- PMCID: PMC11839304
- DOI: 10.1016/j.celrep.2024.115165
The adiponectin-PPARγ axis in hepatic stellate cells regulates liver fibrosis
Abstract
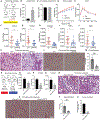
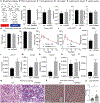
Hepatic stellate cells (HSCs) are key drivers of local fibrosis. Adiponectin, conventionally thought of as an adipokine, is also expressed in quiescent HSCs. However, the impact of its local expression on the progression of liver fibrosis remains unclear. We recently generated a transgenic mouse line (Lrat-rtTA) that expresses the doxycycline-responsive transcriptional activator rtTA under the control of the HSC-specific lecithin retinol acyltransferase (Lrat) promoter, which enables us to specifically and inducibly overexpress or eliminate genes in these cells. The inducible elimination of HSCs protects mice from methionine/choline-deficient (MCD) diet-induced liver fibrosis, confirming their causal involvement in fibrosis development. We generated HSC-specific adiponectin overexpression and null models that demonstrate that HSC-specific adiponectin overexpression dramatically reduces liver fibrosis, whereas HSC-specific adiponectin elimination accelerates fibrosis progression. We identify a local adiponectin-peroxisome proliferator-activated receptor gamma (PPARγ) axis in HSCs that exerts a marked influence on the progression of local fibrosis, independent of circulating adiponectin derived from adipocytes.
Keywords: CP: Metabolism; PPARγ; adiponectin; liver fibrosis; obesity.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK136619/DK/NIDDK NIH HHS/United States
- R01 AG084646/AG/NIA NIH HHS/United States
- P30 AG013319/AG/NIA NIH HHS/United States
- P01 AG051459/AG/NIA NIH HHS/United States
- R00 AG068239/AG/NIA NIH HHS/United States
- R01 DK138035/DK/NIDDK NIH HHS/United States
- R01 DK099110/DK/NIDDK NIH HHS/United States
- R01 DK131537/DK/NIDDK NIH HHS/United States
- R01 DK055758/DK/NIDDK NIH HHS/United States
- R01 DK127274/DK/NIDDK NIH HHS/United States
- RC2 DK118620/DK/NIDDK NIH HHS/United States
- 855170/AHA/American Heart Association-American Stroke Association/United States
- R01 DK136532/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

