Biochemical and Computational Characterization of Haloalkane Dehalogenase Variants Designed by Generative AI: Accelerating the SN2 Step
- PMID: 39792627
- PMCID: PMC12445222
- DOI: 10.1021/jacs.4c15551
Biochemical and Computational Characterization of Haloalkane Dehalogenase Variants Designed by Generative AI: Accelerating the SN2 Step
Abstract
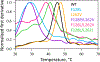
Generative artificial intelligence (AI) models trained on natural protein sequences have been used to design functional enzymes. However, their ability to predict individual reaction steps in enzyme catalysis remains unclear, limiting the potential use of sequence information for enzyme engineering. In this study, we demonstrated that sequence information can predict the rate of the SN2 step of a haloalkane dehalogenase using a generative maximum-entropy (MaxEnt) model. We then designed lower-order protein variants of haloalkane dehalogenase using the model. Kinetic measurements confirmed the successful design of protein variants that enhance catalytic activity, above that of the wild type, in the overall reaction and in particular in the SN2 step. On the simulation side, we provided molecular insights into these designs for the SN2 step using the empirical valence bond (EVB) and metadynamics simulations. The EVB calculations showed activation barriers consistent with experimental reaction rates, while examining the effect of amino acid replacements on the electrostatic effect on the activation barrier and the consequence of water penetration, as well as the extent of ground state destabilization/stabilization. Metadynamics simulations emphasize the importance of the substrate positioning in enzyme catalysis. Overall, our AI-guided approach successfully enabled the design of a variant with a faster rate for the SN2 step than the wild-type enzyme, despite haloalkane dehalogenase being extensively optimized through natural evolution.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

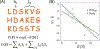
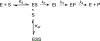


References
-
- Yang KK; Wu Z; Arnold FH Machine-Learning-Guided Directed Evolution for Protein Engineering. Nat. Methods 2019, 16 (8), 687–694. - PubMed
-
- Lin Z; Akin H; Rao R; Hie B; Zhu Z; Lu W; Smetanin N; Verkuil R; Kabeli O; Shmueli Y; dos Santos Costa A; Fazel-Zarandi M; Sercu T; Candido S; Rives A Evolutionary-Scale Prediction of Atomic-Level Protein Structure with a Language Model. Science 2023, 379 (6637), 1123–1130. - PubMed
-
- Bond-Taylor S; Leach A; Long Y; Willcocks CG Deep Generative Modelling: A Comparative Review of VAEs, GANs, Normalizing Flows, Energy-Based and Autoregressive Models. IEEE Trans. Pattern Anal. Mach. Intell 2022, 44 (11), 7327–7347. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

