Molecular dynamics at immune synapse lipid rafts influence the cytolytic behavior of CAR T cells
- PMID: 39792660
- PMCID: PMC11721525
- DOI: 10.1126/sciadv.adq8114
Molecular dynamics at immune synapse lipid rafts influence the cytolytic behavior of CAR T cells
Abstract
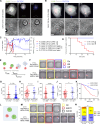
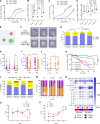
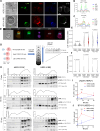
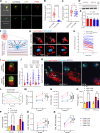
Chimeric antigen receptor T cells (CART) targeting CD19 through CD28.ζ signaling induce rapid lysis of leukemic blasts, contrasting with persistent tumor control exhibited by 4-1BB.ζ-CART. We reasoned that molecular dynamics at the CART immune synapse (CARIS) could explain differences in their tumor rejection kinetics. We observed that CD28.ζ-CART engaged in brief highly lethal CARIS and mastered serial killing, whereas 4-1BB.ζ-CART formed lengthy CARIS and relied on robust expansion and cooperative killing. We analyzed CARIS membrane lipid rafts (mLRs) and found that, upon tumor engagement, CD28.ζ-CAR molecules rapidly but transiently translocated into mLRs, mobilizing the microtubular organizing center and lytic granules to the CARIS. This enabled fast CART recovery and sensitivity to low target site density. In contrast, gradual accumulation of 4-1BB.ζ-CAR and LFA-1 molecules at mLRs built mechanically tonic CARIS mediating chronic Fas ligand-based killing. The differences in CD28.ζ- and 4-1BB.ζ-CARIS dynamics explain the distinct cytolytic behavior of CART and can guide engineering of more adaptive effective cellular products.
Figures

References
-
- Dagher O. K., Schwab R. D., Brookens S. K., Posey A. D. Jr., Advances in cancer immunotherapies. Cell 186, 1814–1814.e1 (2023). - PubMed
-
- Saez-Ibañez A. R., Upadhaya S., Partridge T., Shah M., Correa D., Campbell J., Landscape of cancer cell therapies: Trends and real-world data. Nat. Rev. Drug Discov. 21, 631–632 (2022). - PubMed
-
- Upadhaya S., Yu J. X., Shah M., Correa D., Partridge T., Campbell J., The clinical pipeline for cancer cell therapies. Nat. Rev. Drug Discov. 20, 503–504 (2021). - PubMed
-
- Cappell K. M., Kochenderfer J. N., A comparison of chimeric antigen receptors containing CD28 versus 4-1BB costimulatory domains. Nat. Rev. Clin. Oncol. 18, 715–727 (2021). - PubMed
-
- Lee D. W., Kochenderfer J. N., Stetler-Stevenson M., Cui Y. K., Delbrook C., Feldman S. A., Fry T. J., Orentas R., Sabatino M., Shah N. N., Steinberg S. M., Stroncek D., Tschernia N., Yuan C., Zhang H., Zhang L., Rosenberg S. A., Wayne A. S., Mackall C. L., T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 385, 517–528 (2015). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

