Chromatin conformation, gene transcription, and nucleosome remodeling as an emergent system
- PMID: 39792661
- PMCID: PMC11721585
- DOI: 10.1126/sciadv.adq6652
Chromatin conformation, gene transcription, and nucleosome remodeling as an emergent system
Abstract
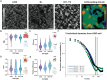
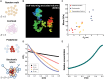
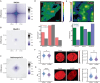
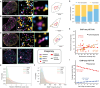
In single cells, variably sized nanoscale chromatin structures are observed, but it is unknown whether these form a cohesive framework that regulates RNA transcription. Here, we demonstrate that the human genome is an emergent, self-assembling, reinforcement learning system. Conformationally defined heterogeneous, nanoscopic packing domains form by the interplay of transcription, nucleosome remodeling, and loop extrusion. We show that packing domains are not topologically associated domains. Instead, packing domains exist across a structure-function life cycle that couples heterochromatin and transcription in situ, explaining how heterochromatin enzyme inhibition can produce a paradoxical decrease in transcription by destabilizing domain cores. Applied to development and aging, we show the pairing of heterochromatin and transcription at myogenic genes that could be disrupted by nuclear swelling. In sum, packing domains represent a foundation to explore the interactions of chromatin and transcription at the single-cell level in human health.
Figures

Similar articles
-
Nuclear blebs are associated with destabilized chromatin-packing domains.J Cell Sci. 2025 Feb 1;138(3):jcs262161. doi: 10.1242/jcs.262161. Epub 2025 Feb 11. J Cell Sci. 2025. PMID: 39878045 Free PMC article.
-
Chromatin organization and behavior in HRAS-transformed mouse fibroblasts.Chromosoma. 2024 Apr;133(2):135-148. doi: 10.1007/s00412-024-00817-x. Epub 2024 Feb 24. Chromosoma. 2024. PMID: 38400910
-
Nucleosome wrapping states encode principles of 3D genome organization.Nat Commun. 2025 Jan 3;16(1):352. doi: 10.1038/s41467-024-54735-8. Nat Commun. 2025. PMID: 39753536 Free PMC article.
-
IKAROS: from chromatin organization to transcriptional elongation control.Cell Death Differ. 2025 Jan;32(1):37-55. doi: 10.1038/s41418-023-01212-2. Epub 2023 Aug 24. Cell Death Differ. 2025. PMID: 37620540 Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
Cited by
-
Nuclear blebs are associated with destabilized chromatin-packing domains.J Cell Sci. 2025 Feb 1;138(3):jcs262161. doi: 10.1242/jcs.262161. Epub 2025 Feb 11. J Cell Sci. 2025. PMID: 39878045 Free PMC article.
-
scNucMap: mapping the nucleosome landscapes at single-cell resolution.Bioinformatics. 2025 Jun 2;41(6):btaf324. doi: 10.1093/bioinformatics/btaf324. Bioinformatics. 2025. PMID: 40424338 Free PMC article.
-
Leveraging chromatin packing domains to target chemoevasion in vivo.Proc Natl Acad Sci U S A. 2025 Jul 29;122(30):e2425319122. doi: 10.1073/pnas.2425319122. Epub 2025 Jul 22. Proc Natl Acad Sci U S A. 2025. PMID: 40694328 Free PMC article.
-
Multiplexed Chromatin Analysis Using Optical Spectroscopic Statistical Nanosensing.ACS Photonics. 2025 Jul 10:10.1021/acsphotonics.5c00311. doi: 10.1021/acsphotonics.5c00311. Online ahead of print. ACS Photonics. 2025. PMID: 40718523 Free PMC article.
References
-
- Shin Y., Brangwynne C. P., Liquid phase condensation in cell physiology and disease. Science 357, eaaf4382 (2017). - PubMed
-
- Arnould C., Rocher V., Saur F., Bader A. S., Muzzopappa F., Collins S., Lesage E., Le Bozec B., Puget N., Clouaire T., Mangeat T., Mourad R., Ahituv N., Noordermeer D., Erdel F., Bushell M., Marnef A., Legube G., Chromatin compartmentalization regulates the response to DNA damage. Nature 623, 183–192 (2023). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

