Perivascular cells function as key mediators of mechanical and structural changes in vascular capillaries
- PMID: 39792671
- PMCID: PMC11721577
- DOI: 10.1126/sciadv.adp3789
Perivascular cells function as key mediators of mechanical and structural changes in vascular capillaries
Abstract
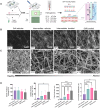
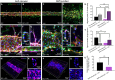
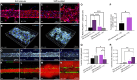
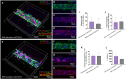
A hallmark of chronic and inflammatory diseases is the formation of a fibrotic and stiff extracellular matrix (ECM), typically associated with abnormal, leaky microvascular capillaries. Mechanisms explaining how the microvasculature responds to ECM alterations remain unknown. Here, we used a microphysiological model of capillaries on a chip mimicking the characteristics of healthy or fibrotic collagen to test the hypothesis that perivascular cells mediate the response of vascular capillaries to mechanical and structural changes in the human ECM. Capillaries engineered in altered fibrotic collagen had abnormal migration of perivascular cells, reduced pericyte differentiation, increased leakage, and higher regulation of inflammatory/remodeling genes, all regulated via NOTCH3, a known mediator of endothelial-perivascular cell communication. Capillaries engineered either with endothelial cells alone or with perivascular cells silenced for NOTCH3 expression showed a minimal response to ECM alterations. These findings reveal a previously unknown mechanism of vascular response to changes in the ECM in health and disease.
Figures

References
-
- Armulik A., Genove G., Betsholtz C., Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 21, 193–215 (2011). - PubMed
-
- von Tell D., Armulik A., Betsholtz C., Pericytes and vascular stability. Exp. Cell Res. 312, 623–629 (2006). - PubMed
-
- Engler A. J., Sen S., Sweeney H. L., Discher D. E., Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006). - PubMed
-
- Discher D. E., Janmey P., Wang Y.-L., Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 (2005). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

