Long, Synthetic Staphylococcus aureus Type 8 Capsular Oligosaccharides Reveal Structural Epitopes for Effective Immune Recognition
- PMID: 39792791
- PMCID: PMC11760181
- DOI: 10.1021/jacs.4c16118
Long, Synthetic Staphylococcus aureus Type 8 Capsular Oligosaccharides Reveal Structural Epitopes for Effective Immune Recognition
Abstract
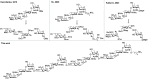
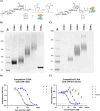
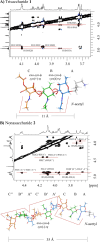
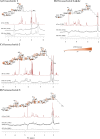
Staphylococcus aureus is a Gram-positive bacterium that is responsible for severe nosocomial infections. The rise of multidrug-resistant strains, which can pose significant health threats, prompts the development of new treatment interventions, and much attention has been directed at the development of prophylactic and therapeutic vaccination strategies. Capsular polysaccharides (CPs) are key protective elements of the S. aureus cell wall and have been proposed as promising candidate antigens. Thirteen different CP serotypes have been identified to date, of which types 5 and 8 are the most prominent. CP8 is composed of trisaccharide repeating units that are built up from an N-acetyl-4-O-acetyl-β-d-mannosaminuronic acid, that carries a C-4-O-acetyl, an N-acetyl-α-d-fucosamine, and an N-acetyl-α-l-fucosamine. Synthetic oligosaccharides are valuable tools to unravel the immunogenicity of bacterial oligosaccharides at the molecular level. However, the rare monosaccharides, cis-glycosidic linkages, and O-acetylation represent significant challenges for the synthesis of CP8 fragments. Here the stereoselective assembly of well-defined CP8 fragments, comprising a trimer, hexamer, nonamer, and dodecamer, is presented. This is the first time that fragments larger than a single repeating trisaccharide, which has been proven to be insufficient for antigenic activity, have been assembled. Structural studies have revealed a linear conformation for the oligosaccharides, with each trisaccharide repeat tilted ∼90° with respect to the flanking repeats, which is stabilized by the acetyl groups that prevent rotation around the glycosidic linkages. The N-acetyl groups in each repeating unit point in the same direction, generating a hydrophobic flank in the trisaccharide repeats. We applied the oligomers to generate model glycoconjugate vaccine modalities, which we then used to raise anti-CP8 antibodies. The antibody interaction and immunization studies have revealed a clear length dependent structure-activity relationship for the oligosaccharides, with an oligosaccharide of at least three repeating units required for an adequate immune response.
Conflict of interest statement
The authors declare the following competing financial interest(s): FC, LDB, MRR, and RA are employees of the GSK group of companies.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

