A Susceptible Cell-Selective Delivery (SCSD) of mRNA-Encoded Cas13d Against Influenza Infection
- PMID: 39792803
- PMCID: PMC11884569
- DOI: 10.1002/advs.202414651
A Susceptible Cell-Selective Delivery (SCSD) of mRNA-Encoded Cas13d Against Influenza Infection
Abstract
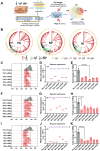
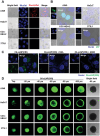
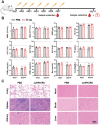
To bolster the capacity for managing potential infectious diseases in the future, it is critical to develop specific antiviral drugs that can be rapidly designed and delivered precisely. Herein, a CRISPR/Cas13d system for broad-spectrum targeting of influenza A virus (IAV) from human, avian, and swine sources is designed, incorporating Cas13d mRNA and a tandem CRISPR RNA (crRNA) specific for the highly conserved regions of viral polymerase acidic (PA), nucleoprotein (NP), and matrix (M) gene segments, respectively. Given that the virus targets cells with specific receptors but is not limited to a single organ, a Susceptible Cell Selective Delivery (SCSD) system is developed by modifying a lipid nanoparticle with a peptide mimicking the function of the hemagglutinin of influenza virus to target sialic acid receptors. The SCSD system can precisely deliver an all-RNA-based CRISPR/Cas13d system into potentially infected cells. This drug is shown to reduce the viral load in the lungs by 2.37 log10 TCID50 mL-1 and protect 100% of mice from lethal influenza infection. The SCSD-based CRISPR/Cas13d system shows promise for the flexible and efficient therapy of infections caused by rapidly evolving and novel viruses.
Keywords: Cas13d mRNA; influenza viruses; susceptible cell‐selective delivery.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Lu L., Su S., Yang H., Jiang S., Cell 2021, 184, 1604. - PubMed
-
- Taubenberger J. K., Reid A. H., Lourens R. M., Wang R., Jin G., Fanning T. G., Nature 2005, 437, 889. - PubMed
-
- Yang R., Sun H., Gao F., Luo K., Huang Z., Tong Q., Song H., Han Q., Liu J., Lan Y., Qi J., Li H., Chen S., Xu M., Qiu J., Zeng G., Zhang X., Huang C., Pei R., Zhan Z., Ye B., Guo Y., Zhou Y., Ye W., Yao D., Ren M., Li B., Yang J., Wang Y., Pu J., et al., Lancet Microbe 2022, 3, e824. - PubMed
-
- Gu C., Maemura T., Guan L., Eisfeld A. J., Biswas A., Kiso M., Uraki R., Ito M., Trifkovic S., Wang T., Babujee L., Presler R. Jr., Dahn R., Suzuki Y., Halfmann P. J., Yamayoshi S., Neumann G., Kawaoka Y., Nature 2024, 636, 711. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
