Epcoritamab plus GemOx in transplant-ineligible relapsed/refractory DLBCL: results from the EPCORE NHL-2 trial
- PMID: 39792928
- PMCID: PMC12000653
- DOI: 10.1182/blood.2024026830
Epcoritamab plus GemOx in transplant-ineligible relapsed/refractory DLBCL: results from the EPCORE NHL-2 trial
Abstract
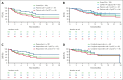
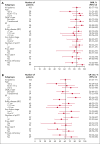
Patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) have poor outcomes (complete response [CR] rates with standard salvage therapy gemcitabine plus oxaliplatin [GemOx], ∼30%; median overall survival [OS], 10 to 13 months). Patients with refractory disease fare worse (CR rate with salvage therapy, 7%; median OS, 6 months). Epcoritamab, a CD3×CD20 bispecific antibody approved for R/R DLBCL after ≥2 therapy lines, has shown promising safety and efficacy in various combinations. We report results from the phase 1b/2 EPCORE NHL-2 trial evaluating epcoritamab plus GemOx in autologous stem cell transplant (ASCT)-ineligible R/R DLBCL. Patients received 48 mg subcutaneous epcoritamab after 2 step-up doses until progression or unacceptable toxicity; GemOx was given once every 2 weeks for 8 doses. The primary end point was overall response rate (ORR). As of 15 December 2023, 103 patients were enrolled (median follow-up, 13.2 months; median age, 72 years). Patients had challenging-to-treat disease: ≥2 prior therapy lines, 62%; prior chimeric antigen receptor T-cell therapy, 28%; primary refractory disease, 52%; refractory to last therapy, 70%. ORR and CR rate were 85% and 61%, respectively. Median duration of CR and OS were 23.6 and 21.6 months, respectively. Common treatment-emergent adverse events were cytopenias and cytokine release syndrome (CRS). CRS events had predictable timing, were primarily low grade (52% overall, 1% grade 3), and resolved without leading to discontinuation. Epcoritamab plus GemOx yielded deep, durable responses and favorable long-term outcomes in ASCT-ineligible R/R DLBCL. This trial was registered at www.clinicaltrials.gov as #NCT04663347.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: J.D.B. reports advisory role for ADC Therapeutics, Epizyme, and Seagen; and research funding from Bristol Myers Squibb, Genentech, Gilead/Kite, and Merck. J.J. reports consultancy role for AbbVie, Gilead, Incyte, Orion, Roche, and Sobi. D.B. reports consultancy role for AbbVie, Bristol Myers Squibb, Genmab, Gilead, MorphoSys, Roche, Sobi, and Takeda; honoraria from AbbVie, Gilead, Janssen, MorphoSys, Roche, and Takeda; and travel accommodations/expenses from AbbVie, Recordati, and Roche. M.T. reports consultancy role for AbbVie, Amgen, Bristol Myers Squibb, Genmab, Gilead, Incyte, Janssen, MorphoSys, Novartis, Roche, Sobi, and Takeda; honoraria from AbbVie, Amgen, Bristol Myers Squibb, Gilead, Janssen, MorphoSys, Novartis, Roche, and Takeda; and travel accommodations/expenses from AbbVie, Bristol Myers Squibb, Gilead, Janssen, Roche, and Takeda. U.V. reports advisory role for AbbVie, Genmab, Gilead, Incyte, and Regeneron; and lecture fees from AbbVie, Gilead, Incyte, Janssen, Merck Sharp & Dohme, Regeneron, Roche, and Servier. D.J.L. reports advisory and consultancy roles for Janssen, Lilly, Roche, BeiGene, and Kite. Y.H.K. reports advisory or consultancy roles for AbbVie and ADC Therapeutics; travel expenses from Roche/Genentech; and research funding from AbbVie, AstraZeneca, Lilly/Loxo, Merck, Roche/Genentech, and Xencor. A.S. reports consultancy role for Takeda, Bristol Myers Squibb, Novartis, Janssen, Merck Sharp & Dohme, Amgen, GlaxoSmithKline (GSK), Sanofi, Kite, and Mundipharma; honoraria from Takeda, Bristol Myers Squibb, Novartis, Janssen, Merck Sharp & Dohme, Amgen, GSK, Sanofi, and Kite; membership on an entity’s board of directors or advisory role for Takeda, Bristol Myers Squibb, Novartis, Janssen, Amgen, Bluebird, Sanofi, and Kite; travel expenses from Takeda, Bristol Myers Squibb, and Roche; research funding from Takeda; and speaker’s role for Takeda, Bristol Myers Squibb, Novartis, Janssen, Merck Sharp & Dohme, Amgen, GSK, Sanofi, and Kite. B.E.W. reports consultancy role and research funding from Roche, and research funding from Gilead. P.J.L. reports advisory honoraria from Bristol Myers Squibb, Roche, Takeda, Genmab, AbbVie, Incyte, Regeneron, and Sandoz; and consultancy honoraria from Y-mAbs Therapeutics. T.J. reports current employment at AbbVie. K.K., A.J.S., A.A., L.W., and M.R. report current employment and stock ownership at Genmab. R. Cordoba reports consultancy role for AbbVie, Janssen, AstraZeneca, Kite, Bristol Myers Squibb, Genmab, Roche, Takeda, Kyowa Kirin, BeiGene, and Lilly; speaker’s role for AbbVie, Janssen, AstraZeneca, Kite, Bristol Myers Squibb, Roche, and Takeda; and research funding from Pfizer. The remaining authors declare no competing financial interests.
Figures

Comment in
-
CD3×CD20 bispecifics + chemotherapy: good news for R/R DLBCL.Blood. 2025 Apr 10;145(15):1591-1592. doi: 10.1182/blood.2024028066. Blood. 2025. PMID: 40208652 No abstract available.
References
-
- Al-Hamadani M, Habermann TM, Cerhan JR, Macon WR, Maurer MJ, Go RS. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: a longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol. 2015;90(9):790–795. - PubMed
-
- Cazelles C, Belhadj K, Vellemans H, et al. Rituximab plus gemcitabine and oxaliplatin (R-GemOx) in refractory/relapsed diffuse large B-cell lymphoma: a real-life study in patients ineligible for autologous stem-cell transplantation. Leuk Lymphoma. 2021;62(9):2161–2168. - PubMed
-
- Abramson JS, Ku M, Hertzberg M, et al. Glofitamab plus gemcitabine and oxaliplatin (GemOx) versus rituximab-GemOx for relapsed or refractory diffuse large B-cell lymphoma (STARGLO): a global phase 3, randomised, open-label trial. Lancet. 2024;404(10466):1940–1954. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

