Advancing the Mechanosensitivity of Atropisomeric Diarylethene Mechanophores through a Lever-Arm Effect
- PMID: 39793028
- PMCID: PMC11760174
- DOI: 10.1021/jacs.4c13480
Advancing the Mechanosensitivity of Atropisomeric Diarylethene Mechanophores through a Lever-Arm Effect
Abstract
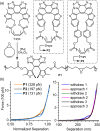
Understanding structure-mechanical activity relationships (SMARs) in polymer mechanochemistry is essential for the rational design of mechanophores with desired properties, yet SMARs in noncovalent mechanical transformations remain relatively underexplored. In this study, we designed a subset of diarylethene mechanophores based on a lever-arm hypothesis and systematically investigated their mechanical activity toward a noncovalent-yet-chemical conversion of atropisomer stereochemistry. Results from Density functional theory (DFT) calculations, single-molecule force spectroscopy (SMFS) measurements, and ultrasonication experiments collectively support the lever-arm hypothesis and confirm the exceptional sensitivity of chemo-mechanical coupling in these atropisomers. Notably, the transition force for the diarylethene M3 featuring extended 5-phenylbenzo[b]thiophene aryl groups is determined to be 131 pN ± 4 pN by SMFS. This value is lower than those typically recorded for other mechanically induced chemical processes, highlighting its exceptional sensitivity to low-magnitude forces. This work contributes a fundamental understanding of chemo-mechanical coupling in atropisomeric configurational mechanophores and paves the way for designing highly sensitive mechanochemical processes that could facilitate the study of nanoscale mechanical behaviors across scientific disciplines.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Berkowski K. L.; Potisek S. L.; Hickenboth C. R.; Moore J. S. Ultrasound-Induced Site-Specific Cleavage of Azo-Functionalized Poly(ethylene glycol). Macromolecules 2005, 38 (22), 8975–8978. 10.1021/ma051394n. - DOI
-
- Davis D. A.; Hamilton A.; Yang J.; Cremar L. D.; Van Gough D.; Potisek S. L.; Ong M. T.; Braun P. V.; Martínez T. J.; White S. R.; Moore J. S.; Sottos N. R. Force-Induced Activation of Covalent Bonds in Mechanoresponsive Polymeric Materials. Nature 2009, 459 (7243), 68–72. 10.1038/nature07970. - DOI - PubMed
LinkOut - more resources
Full Text Sources

