Hydroxychloroquine prevents resistance and potentiates the antitumor effect of SHP2 inhibition in NF1-associated malignant peripheral nerve sheath tumors
- PMID: 39793045
- PMCID: PMC11725864
- DOI: 10.1073/pnas.2407745121
Hydroxychloroquine prevents resistance and potentiates the antitumor effect of SHP2 inhibition in NF1-associated malignant peripheral nerve sheath tumors
Abstract
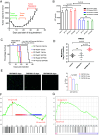
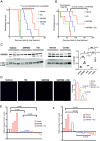
Malignant peripheral nerve sheath tumors (MPNSTs) are aggressive sarcomas and the primary cause of mortality in patients with neurofibromatosis type 1 (NF1). These malignancies develop within preexisting benign lesions called plexiform neurofibromas (PNs). PNs are solely driven by biallelic NF1 loss eliciting RAS pathway activation, and they respond favorably to MEK inhibitor therapy. MPNSTs harbor additional mutations and respond poorly to MEK inhibition. Our analysis of genetically engineered and orthotopic patient-derived xenograft MPNST models indicates that MEK inhibition has poor antitumor efficacy. By contrast, upstream inhibition of RAS through the protein-tyrosine phosphatase SHP2 reduced downstream signaling and suppressed NF1 MPNST growth, although resistance eventually emerged. To investigate possible mechanisms of acquired resistance, kinomic analyses of resistant tumors were performed, and data analysis identified enrichment of activated autophagy pathway protein kinases. Combining SHP2 inhibition with hydroxychloroquine (HQ) resulted in durable responses in NF1 MPNSTs in both genetic and orthotopic xenograft mouse models. Our studies could be rapidly translated into a clinical trial to evaluate SHP2 inhibition in conjunction with HQ as a unique treatment approach for NF1 MPNSTs.
Keywords: MPNST; NF1; SHP2 inhibition; autophagy; hydroxychloroquine.
Conflict of interest statement
Competing interests statement:B.G.N. reports personal fees and other support from Northern Biologics, Ltd, Navire Pharma, Lighthorse Therapeutics, Aethon Therapeutics and other support from Recursion Pharma, Arvinas, Regeneron, Moderna, Revolution Medicines, Mirati, and Amgen, Inc. outside the submitted work. Clinical trials of the Novartis MEK inhibitor Trametinib in conjunction with HQ and Verastem Oncology MEK inhibitor Avutometinib are underway at MSKCC. Clinical trials of the Novartis SHP2 inhibitor TNO155 and the Amgen (Sotorasib), Mirati (Adagrasib), and Revolution Medicines (RMC-6236) KRAS inhibitors have been performed or are under way at PCC. No disclosures were reported by the other authors. The authors declare that they have no affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this manuscript.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

