Tracheal tuft cells release ATP and link innate to adaptive immunity in pneumonia
- PMID: 39794305
- PMCID: PMC11724094
- DOI: 10.1038/s41467-025-55936-5
Tracheal tuft cells release ATP and link innate to adaptive immunity in pneumonia
Abstract
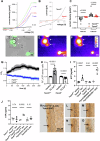
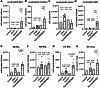
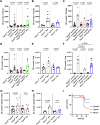
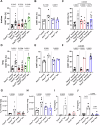
Tracheal tuft cells shape immune responses in the airways. While some of these effects have been attributed to differential release of either acetylcholine, leukotriene C4 and/or interleukin-25 depending on the activating stimuli, tuft cell-dependent mechanisms underlying the recruitment and activation of immune cells are incompletely understood. Here we show that Pseudomonas aeruginosa infection activates mouse tuft cells, which release ATP via pannexin 1 channels. Taste signaling through the Trpm5 channel is essential for bacterial tuft cell activation and ATP release. We demonstrate that activated tuft cells recruit dendritic cells to the trachea and lung. ATP released by tuft cells initiates dendritic cell activation, phagocytosis and migration. Tuft cell stimulation also involves an adaptive immune response through recruitment of IL-17A secreting T helper cells. Collectively, the results provide a molecular framework defining tuft cell dependent regulation of both innate and adaptive immune responses in the airways to combat bacterial infection.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
-
- Hollenhorst, M. I. & Krasteva-Christ, G. Chemosensory cells in the respiratory tract as crucial regulators of innate immune responses. J. Physiol.601, 1555–1572 (2023). - PubMed
-
- Hollenhorst, M. I. et al. Tracheal brush cells release acetylcholine in response to bitter tastants for paracrine and autocrine signaling. FASEB J.34, 316–332 (2020). - PubMed
MeSH terms
Substances
Grants and funding
- KR 4338/1-2 (to GKC)/Deutsche Forschungsgemeinschaft (German Research Foundation)
- SFB TRR152 P22 (to GKC), P11 (to UB), Z02 (to VF and UB), P15 (to VC and TG)/Deutsche Forschungsgemeinschaft (German Research Foundation)
- SFB 894 P2 (to UB)/Deutsche Forschungsgemeinschaft (German Research Foundation)
- Research Training Group 2338 (to TG)/Deutsche Forschungsgemeinschaft (German Research Foundation)
- R21 ES036349/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

