Gut microbes modulate the effects of the flavonoid quercetin on atherosclerosis
- PMID: 39794320
- PMCID: PMC11723976
- DOI: 10.1038/s41522-024-00626-1
Gut microbes modulate the effects of the flavonoid quercetin on atherosclerosis
Abstract
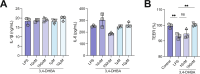
Gut bacterial metabolism of dietary flavonoids results in the production of a variety of phenolic acids, whose contributions to health remain poorly understood. Here, we show that supplementation with the commonly consumed flavonoid quercetin impacted gut microbiome composition and resulted in a significant reduction in atherosclerosis burden in conventionally raised (ConvR) Apolipoprotein E (ApoE) knockout (KO) mice but not in germ-free (GF) ApoE KO mice. Metabolomic analysis revealed that consumption of quercetin significantly increased plasma levels of benzoylglutamic acid, 3,4 dihydroxybenzoic acid (3,4-DHBA) and its sulfate-conjugated form in ConvR mice, but not in GF mice supplemented with the flavonoid. Levels of these metabolites were negatively associated with atherosclerosis burden. Furthermore, we show that 3,4-DHBA prevented lipopolysaccharide (LPS)-induced decrease in transendothelial electrical resistance (TEER). These results suggest that the effects of quercetin on atherosclerosis are influenced by gut microbes and are potentially mediated by bacterial metabolites derived from the flavonoid.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: F.B. is co-founder and shareholder of Roxbiosens Inc and Implexion Pharma AB, receives research funding from Biogaia AB, and is a member of the scientific advisory board of Bactolife A/S.
Figures



Update of
-
Gut microbiota and diet matrix modulate the effects of the flavonoid quercetin on atherosclerosis.Res Sq [Preprint]. 2023 Jan 10:rs.3.rs-2431147. doi: 10.21203/rs.3.rs-2431147/v1. Res Sq. 2023. Update in: NPJ Biofilms Microbiomes. 2025 Jan 10;11(1):12. doi: 10.1038/s41522-024-00626-1. PMID: 36712088 Free PMC article. Updated. Preprint.
References
-
- Kolodziejczyk, A. A., Zheng, D. & Elinav, E. Diet–microbiota interactions and personalized nutrition. Nat. Rev. Microbiol.17, 742–753 (2019). - PubMed
MeSH terms
Substances
Grants and funding
- T32 HL007936/HL/NHLBI NIH HHS/United States
- HL148577/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL148577/HL/NHLBI NIH HHS/United States
- HL007936/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- HL144651/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

