GC1126A, a novel ADAMTS13 mutein, evades autoantibodies in immune-mediated thrombotic thrombocytopenic purpura
- PMID: 39794345
- PMCID: PMC11723924
- DOI: 10.1038/s41598-024-80674-x
GC1126A, a novel ADAMTS13 mutein, evades autoantibodies in immune-mediated thrombotic thrombocytopenic purpura
Abstract
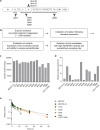
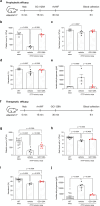
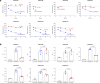
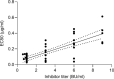
Immune-mediated thrombotic thrombocytopenic purpura (iTTP) is a rare and life-threatening blood disorder characterized by the formation of blood clots in small blood vessels. It is caused by antibodies targeting the A disintegrin and metalloprotease with thrombospondin type 1 repeats, member 13 (ADAMTS13), which plays a role in cleaving von Willebrand factor. Most patients with iTTP have autoantibodies against specific domains of the ADAMTS13 protein, particularly the cysteine-rich and spacer domains. This study aimed to identify ADAMTS13 muteins that are resistant to autoantibodies and maintain their enzymatic activity. A panel of muteins was generated using rational and random mutagenesis methods and screened for autoantibody binding and ADAMTS13 activity. The selected muteins were assessed for pharmacodynamic biomarkers and pharmacokinetic profiles in the iTTP-mimic and wild-type mice, respectively. GC1126A was the most effective variant for escaping autoantibodies and had a longer half-life than the wild-type ADAMTS13 fragment (MDTCS). In the iTTP-mimic mouse model, GC1126A treatment significantly improved platelet counts, lactate dehydrogenase levels, and ADAMTS13 residual activity. In addition, GC1126A outperformed recombinant human wild-type ADAMTS13 (rh WT-ADAMTS13) and caplacizumab in terms of platelet recovery and sustained effectiveness. Results from the ex vivo study using plasma from patients with iTTP showed that GC1126A exhibited higher residual activity than rh WT-ADAMTS13, particularly in patients with high autoantibody titers. These findings suggest that GC1126A could be a promising new treatment option for patients with iTTP.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: HK, GC, SK, YK, YL, CL, and HJN are inventors on a patent application regarding GC1126A. HK, GC, SK, JMP, YK, YL, CL, and HJN were employed by GC Biopharma Corp. The remaining authors declare no competing financial interests.
Figures


Similar articles
-
Anti-ADAMTS13 Autoantibodies against Cryptic Epitopes in Immune-Mediated Thrombotic Thrombocytopenic Purpura.Thromb Haemost. 2018 Oct;118(10):1729-1742. doi: 10.1055/s-0038-1669459. Epub 2018 Sep 20. Thromb Haemost. 2018. PMID: 30235483
-
N-glycan-mediated shielding of ADAMTS13 prevents binding of pathogenic autoantibodies in immune-mediated TTP.Blood. 2021 May 13;137(19):2694-2698. doi: 10.1182/blood.2020007972. Blood. 2021. PMID: 33544829 Free PMC article.
-
Challenges in managing iTTP: insights into ADAMTS13 inhibitor boosting during caplacizumab therapy.Ann Hematol. 2025 Mar;104(3):1507-1514. doi: 10.1007/s00277-025-06318-w. Epub 2025 Mar 19. Ann Hematol. 2025. PMID: 40105947 Free PMC article.
-
Refining the standard of care in immune thrombotic thrombocytopenic purpura.Clin Adv Hematol Oncol. 2024 Oct;22(8):381-391. Clin Adv Hematol Oncol. 2024. PMID: 39356816 Review.
-
ADAMTS13 conformations and mechanism of inhibition in immune thrombotic thrombocytopenic purpura.J Thromb Haemost. 2022 Oct;20(10):2197-2203. doi: 10.1111/jth.15822. Epub 2022 Aug 3. J Thromb Haemost. 2022. PMID: 35842925 Free PMC article. Review.
References
-
- Lancellotti, S. & De Cristofaro, R. Structure and proteolytic properties of ADAMTS13, a metalloprotease involved in the pathogenesis of thrombotic microangiopathies. Prog. Mol. Biol. Transl. Sci.99, 105–144. 10.1016/B978-0-12-385504-6.00003-8 (2011). - PubMed
-
- Joly, B. S., Coppo, P. & Veyradier, A. An update on pathogenesis and diagnosis of thrombotic thrombocytopenic purpura. Expert Rev. Hematol.12(6), 383–395. 10.1080/17474086.2019.1611423 (2019). - PubMed
-
- Joly, B. S., Coppo, P. & Veyradier, A. Thrombotic thrombocytopenic purpura. Blood129(21), 2836–2846. 10.1182/blood-2016-10-709857 (2017). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

