Tenascin-C promotes bone regeneration via inflammatory macrophages
- PMID: 39794452
- PMCID: PMC11982535
- DOI: 10.1038/s41418-024-01429-9
Tenascin-C promotes bone regeneration via inflammatory macrophages
Abstract
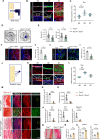
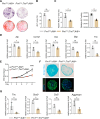
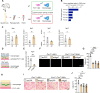
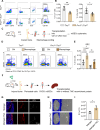
During the early stage of tissue injury, macrophages play important roles in the activation of stem cells for further regeneration. However, the regulation of macrophages during bone regeneration remains unclear. Here, the extracellular matrix (ECM) tenascin-C (TNC) is found to express in the periosteum and recruit inflammatory macrophages. TNC-deficiency in the periosteum delays bone repair. Transplantation of macrophages derived from injured periosteum is able to rescue the decreased skeletal stem cells and impaired bone regeneration caused by TNC deficiency. The cell communication analysis identifies ITGA7 as a TNC receptor contributing to the recruitment of inflammatory macrophages. TNC expression declines in aged mice and the exogenous delivery of TNC significantly promotes bone regeneration after aging through the recruitment of macrophages. Taken together, this study reveals the regulation of macrophage recruitment and its function in the activation of skeletal stem cells after bone injury, providing a strategy to accelerate bone regeneration by TNC delivery.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

