Subcutaneous weekly semaglutide with automated insulin delivery in type 1 diabetes: a double-blind, randomized, crossover trial
- PMID: 39794615
- PMCID: PMC12003151
- DOI: 10.1038/s41591-024-03463-z
Subcutaneous weekly semaglutide with automated insulin delivery in type 1 diabetes: a double-blind, randomized, crossover trial
Abstract
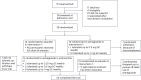
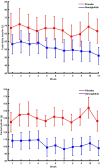
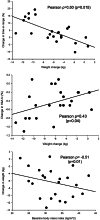
Efforts to improve glycemic control in type 1 diabetes are ongoing. We performed a randomized, double-blind, crossover trial to assess semaglutide as adjunct to automated insulin delivery therapy in adults with type 1 diabetes. At each intervention, participants were titrated up to 1 mg or the maximum tolerated dose of semaglutide or placebo over 11 weeks, followed by the use of an automated insulin delivery system for 4 weeks. The primary outcome was the percentage of time spent in the target glucose range of 3.9-10.0 mmol l-1 during the last 4 weeks of each intervention. Twenty-eight participants were randomized and 24 completed the trial. The primary endpoint was met. Compared to placebo, semaglutide increased time in the target range by a mean 4.8 (s.d. = 7.6) percentage points (P = 0.006), without increasing the time spent below 3.9 mmol l-1 (P = 0.19) or below 3.0 mmol l-1 (P = 0.65). While no diabetic ketoacidosis or severe hypoglycemia occurred during any of the interventions, there were two episodes of recurrent euglycemic ketosis without acidosis during semaglutide use. We conclude that semaglutide improves glycemic control with automated insulin delivery compared to placebo. ClinicalTrials.gov registration: NCT05205928.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: M.-R.P. has received speaker honoraria from Medtronic Diabetes Canada and Abbott Diabetes Care. M.A.T. has received speaker honoraria from AstraZeneca, Bausch Health, Boehringer Ingelheim, Eli Lilly, Janssen, Novo Nordisk and Sanofi. A.H. has acted as a consultant for Eli Lilly and has received drugs, supplies, equipment and other in-kind support from Tandem, Adocia, Dexcom, Eli Lilly and Ypsomed. A.K., W.A. and A.J. declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

