Autologous macrophage therapy for liver cirrhosis: a phase 2 open-label randomized controlled trial
- PMID: 39794616
- PMCID: PMC11922741
- DOI: 10.1038/s41591-024-03406-8
Autologous macrophage therapy for liver cirrhosis: a phase 2 open-label randomized controlled trial
Abstract
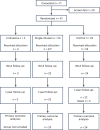
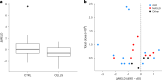
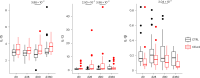
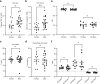
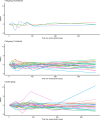
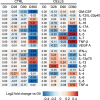
Cirrhosis is a major cause of morbidity and mortality; however, there are no approved therapies except orthotopic liver transplantation. Preclinical studies showed that bone-marrow-derived macrophage injections reduce inflammation, resolve fibrosis and stimulate liver regeneration. In a multicenter, open-label, parallel-group, phase 2 randomized controlled trial ( ISRCTN10368050 ) in n = 51 adult patients with compensated cirrhosis and Model for End-Stage Liver Disease (MELD) score ≥10 and ≤17, we evaluated the efficacy of autologous monocyte-derived macrophage therapy (n = 27) compared to standard medical care (n = 24). The primary endpoint was the difference in baseline to day 90 change in MELD score (ΔMELD) between treatment and control groups (ΔΔMELD). Secondary endpoints included adverse clinical outcomes, non-invasive fibrosis biomarkers and health-related quality of life (HRQoL) at 90 d, 180 d and 360 d. The ΔΔMELD between day 0 and day 90 in the treatment group compared to controls was -0.87 (95% confidence interval: -1.79, 0.0; P = 0.06); therefore, the primary endpoint was not met. During 360-d follow-up, five of 24 participants in the control group developed a total of 10 severe adverse events, four of which were liver related, and three deaths (two liver related), whereas no liver-related severe adverse events or deaths occurred in the treatment group. Although no differences were observed in biomarkers or HRQoL, exploratory analysis showed anti-inflammatory serum cytokine profiles after macrophage infusion. This study reinforces the safety and potential efficacy of macrophage therapy in cirrhosis, supporting further investigation.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: P.N.B. has received educational honoraria from Takeda and has served as a consultant to Resolution Therapeutics. I.M.P. has received speaker fees from Advanz Pharma. J.F.D. has received research grants and lecture honoraria from Merck Sharp & Dohme, AbbVie and Gilead. A.M.K. is a consultant to Resolution Therapeutics. J.D.M.C. is a founder of and adviser to Resolution Therapeutics. J.A.F. serves as a consultant or advisory board member for Stellaris, Kynos Therapeutics, Resolution Therapeutics, Ipsen, River 2 Renal Corp., Stimuliver, Global Clinical Trial Partners and Guidepoint and has received speaker fees from HistoIndex and research grant funding from GlaxoSmithKline, Intercept Pharmaceuticals and Genentech. S.J.F. is a founder of and adviser to Resolution Therapeutics and an adviser to Cytotheryx. The other authors declare no competing interests.
Figures

References
-
- Devarbhavi, H. et al. Global burden of liver disease: 2023 update. J. Hepatol.79, 516–537 (2023). - PubMed
-
- Brennan, P. N. et al. Progress is impossible without change: understanding the evolving nomenclature of steatotic liver disease and its effect on hepatology practice. Lancet Gastroenterol. Hepatol.9, 577–582 (2024). - PubMed
-
- Pellicoro, A., Ramachandran, P., Iredale, J. P. & Fallowfield, J. A. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat. Rev. Immunol.14, 181–194 (2014). - PubMed
-
- D’Amico, G., Garcia-Tsao, G. & Pagliaro, L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J. Hepatol.44, 217–231 (2006). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

