Effect of SNORD113-3/ADAR2 on glycolipid metabolism in glioblastoma via A-to-I editing of PHKA2
- PMID: 39794701
- PMCID: PMC11724473
- DOI: 10.1186/s11658-024-00680-9
Effect of SNORD113-3/ADAR2 on glycolipid metabolism in glioblastoma via A-to-I editing of PHKA2
Abstract
Background: Glioblastoma multiforme (GBM) is a highly aggressive brain tumor, characterized by its poor prognosis. Glycolipid metabolism is strongly associated with GBM development and malignant behavior. However, the precise functions of snoRNAs and ADARs in glycolipid metabolism within GBM cells remain elusive. The objective of the present study is to delve into the underlying mechanisms through which snoRNAs and ADARs exert regulatory effects on glycolipid metabolism in GBM cells.
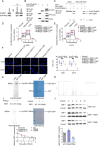
Methods: RNA immunoprecipitation and RNA pull-down experiments were conducted to verify the homodimerization of ADAR2 by SNORD113-3, and Sanger sequencing and Western blot experiments were used to detect the A-to-I RNA editing of PHKA2 mRNA by ADAR2. Furthermore, the phosphorylation of EBF1 was measured by in vitro kinase assay. Finally, in vivo studies using nude mice confirmed that SNORD113-3 and ADAR2 overexpression, along with PHKA2 knockdown, could suppress the formation of subcutaneous xenograft tumors and improve the outcome of tumor-bearing nude mice.
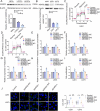
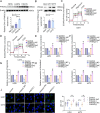
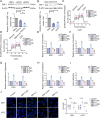
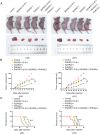
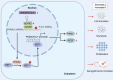
Results: We found that PHKA2 in GBM significantly promoted glycolipid metabolism, while SNORD113-3, ADAR2, and EBF1 significantly inhibited glycolipid metabolism. SNORD113-3 promotes ADAR2 protein expression by promoting ADAR2 homodimer formation. ADAR2 mediates the A-to-I RNA editing of PHKA2 mRNA. Mass spectrometry analysis and in vitro kinase testing revealed that PHKA2 phosphorylates EBF1 on Y256, reducing the stability and expression of EBF1. Furthermore, direct binding of EBF1 to PKM2 and ACLY promoters was observed, suggesting the inhibition of their expression by EBF1. These findings suggest the existence of a SNORD113-3/ADAR2/PHKA2/EBF1 pathway that collectively regulates the metabolism of glycolipid and the growth of GBM cells. Finally, in vivo studies using nude mice confirmed that knockdown of PHKA2, along with overexpression of SNORD113-3 and ADAR2, could obviously suppress GBM subcutaneous xenograft tumor formation and improve the outcome of those tumor-bearing nude mice.
Conclusions: Herein, we clarified the underlying mechanism involving the SNORD113-3/ADAR2/PHKA2/EBF1 pathway in the regulation of GBM cell growth and glycolipid metabolism. Our results provide a framework for the development of innovative therapeutic interventions to improve the prognosis of patients with GBM.
Keywords: A-to-I RNA editing; ADAR2; Glioma; Glycolipid metabolism; Phosphorylation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All patients have provided their informed consent with ethical approval granted by the Ethics Committee of the Affiliated Hospital of China Medical University. Similarly, the animal experiments conducted have been authorized by the Ethics Committee of the Affiliated Hospital of China Medical University. Competing interests: The authors declare that they have no competing interests.
Figures




References
-
- Janjua TI, Rewatkar P, Ahmed-Cox A, Saeed I, Mansfeld FM, Kulshreshtha R, Kumeria T, Ziegler DS, Kavallaris M, Mazzieri R, et al. Frontiers in the treatment of glioblastoma: past, present and emerging. Adv Drug Deliv Rev. 2021;171:108–38. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

