Gastrostomy tube feeding in children: a single-center experience
- PMID: 39794725
- PMCID: PMC11720959
- DOI: 10.1186/s12876-024-03582-4
Gastrostomy tube feeding in children: a single-center experience
Abstract
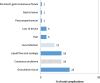
Background: Despite the widespread use of percutaneous endoscopic gastrostomy (PEG) in pediatric populations, there is a paucity of data on the indications and outcomes of this procedure in Switzerland. This manuscript presents our experience with PEG indication, outcomes, and related complications in children.
Methods: This single-center retrospective study included patients < 18 years old who underwent PEG placement between 2007 and 2016. We retrieved demographics, PEG indications, associated comorbidities, pre-placement workup, growth parameters up to 12 months, and associated complications.
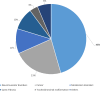
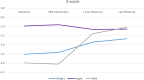
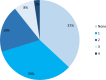
Results: Eighty-one patients were included, with a median age of 7 years. Common indications included inadequate caloric intake (85%), failure to thrive, and feeding difficulties. Neurological conditions (46%) were the most commonly associated comorbidity. Thirty-six patients (44%) underwent a pH study before PEG placement. There were significant increases in z-scores for weight (p < 0.002) and body mass index (p < 0.001) 12 months after PEG placement. Minor complications were relatively frequent (n = 55, 68%), mainly granulation tissue or local erythema. Two patients had major complications.
Conclusion: PEG is a safe technique for providing long-term enteral nutrition in children, with neurological disease being the most common clinical indication. Our experience demonstrated significant weight gain in children after one year of PEG, with frequent but well-controlled complications.
Keywords: Children; Enteral feeding; Malnutrition; Neurological disease; Pediatric; Percutaneous endoscopic gastrostomy.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Human Ethics Committee of the University of Lausanne (protocol 2017 − 00124), Switzerland. Due to the retrospective nature of the study, the need for written informed consent was waived by the Ethics Committee. All procedures were conducted in line with the principles of the Declaration of Helsinki and other applicable ethical standards. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Gauderer MWL, Ponsky JL, Izant RJ. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg. 1980;15:872–5. - PubMed
-
- Homan M, Hauser B, Romano C, Tzivinikos C, Torroni F, Gottrand F, et al. Percutaneous endoscopic gastrostomy in children: an update to the ESPGHAN position paper. J Pediatr Gastroenterol Nutr. 2021;73:415–26. - PubMed
-
- Rutter CE, Yovino S, Taylor R, Wolf J, Cullen K, Ord R, et al. Early PEG Tube Placement improves Nutritional Status and decreases hospitalization in Head and Neck Cancer patients receiving definitive chemoradiation. Int J Radiat Oncol. 2009;75:S132–3.
-
- Imbertson E, Aguirre T, Chen S, Grotts J. Clinical outcomes of In-hospital PEG tube Placement. Am J Gastroenterol. 2013;108:S474.
-
- Fröhlich T, Richter M, Carbon R, Barth B, Köhler H. Review article: percutaneous endoscopic gastrostomy in infants and children. Aliment Pharmacol Ther. 2010;31:788–801. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources