PRMT5 inhibition has a potent anti-tumor activity against adenoid cystic carcinoma of salivary glands
- PMID: 39794830
- PMCID: PMC11724466
- DOI: 10.1186/s13046-024-03270-x
PRMT5 inhibition has a potent anti-tumor activity against adenoid cystic carcinoma of salivary glands
Abstract
Background: Adenoid cystic carcinoma (ACC) is a rare glandular malignancy, commonly originating in salivary glands of the head and neck. Given its protracted growth, ACC is usually diagnosed in advanced stage. Treatment of ACC is limited to surgery and/or adjuvant radiotherapy, which often fails to prevent disease recurrence, and no FDA-approved targeted therapies are currently available. As such, identification of new therapeutic targets specific to ACC is crucial for improved patients' outcomes.
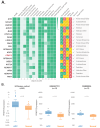
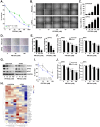
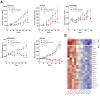
Methods: After thoroughly evaluating the gene expression and signaling patterns characterizing ACC, we applied PandaOmics (an AI-driven software platform for novel therapeutic target discovery) on the unique transcriptomic dataset of 87 primary ACCs. Identifying protein arginine methyl transferase 5 (PRMT5) as a putative candidate with the top-scored druggability, we next determined the applicability of PRMT5 inhibitors (PRT543 and PRT811) using ACC cell lines, organoids, and patient derived xenograft (PDX) models. Molecular changes associated with response to PRMT5 inhibition and anti-proliferative effect of the combination therapy with lenvatinib was then analyzed.
Results: Using a comprehensive AI-powered engine for target identification, PRMT5 was predicted among potential therapeutic target candidates for ACC. Here we show that monotherapy with selective PRMT5 inhibitors induced a potent anti-tumor activity across several cellular and animal models of ACC, which was paralleled by downregulation of genes associated with ACC tumorigenesis, including MYB and MYC (the recognized drivers of ACC progression). Furthermore, as a subset of genes targeted by lenvatinib is upregulated in ACC, we demonstrate that addition of lenvatinib enhanced the growth inhibitory effect of PRMT5 blockade in vitro, suggesting a potential clinical benefit for patients expressing lenvatinib favorable molecular profile.
Conclusion: Taken together, our study underscores the role of PRMT5 in ACC oncogenesis and provides a strong rationale for the clinical development of PRMT5 inhibitors as a targeted monotherapy or combination therapy for treatment of patients with this rare disease, based on the analysis of their underlying molecular profile.
Keywords: Adenoid cystic carcinoma (ACC); Organoid models; PandaOmics; Patient derived xenografts (PDXs); Protein arginine methyl transferase 5 (PRMT5); RNA-Seq; Whole exome sequencing.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All human samples used in this study were obtained by the Human Tissue Resource Center at the University of Chicago following informed written consent, under the Institutional Review Boards (IRB)-approved protocols. Consent for publication: Not applicable. Competing interests: VT, KI, PS, and BR are affiliated with Prelude Therapeutics, a clinical-stage biopharmaceutical company designing and developing novel small molecule therapies that target the key drivers of cancer cell growth and resistance. MK, VS, AO, OG, IO, FP, and AZ are affiliated with InSilico Medicine, a company developing an AI-based end-to-end integrated pipeline for drug discovery and development. The remaining authors declare no conflict of interest.
Figures



References
-
- Boyle TAC, Laurent SS, Semus S, Joseph N. Epidemiology of adenoid cystic carcinoma in the United States. J Clin Oncol. 2020;38:e13600–13600.
-
- Ellington CL, et al. Adenoid cystic carcinoma of the head and neck: incidence and survival trends based on 1973–2007 Surveillance, Epidemiology, and end results data. Cancer. 2012;118:4444–51. - PubMed
-
- Fonseca FP, et al. Clinicopathologic analysis of 493 cases of salivary gland tumors in a Southern Brazilian population. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:230–9. - PubMed
-
- Bjørndal K, et al. Salivary adenoid cystic carcinoma in Denmark 1990–2005: outcome and independent prognostic factors including the benefit of radiotherapy. Results of the Danish Head and Neck Cancer Group (DAHANCA). Oral Oncol. 2015;51:1138–42. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

