RNA-Seq and ATAC-Seq Reveal CYP26A1-Mediated Regulation of Retinoic Acid-Induced Meiosis in Chicken Primordial Germ Cells
- PMID: 39794966
- PMCID: PMC11718974
- DOI: 10.3390/ani15010023
RNA-Seq and ATAC-Seq Reveal CYP26A1-Mediated Regulation of Retinoic Acid-Induced Meiosis in Chicken Primordial Germ Cells
Abstract
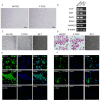
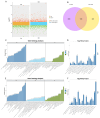
Retinoic acid (RA) plays a critical role in initiating meiosis in primordial germ cells (PGC), yet the specific mechanisms of its interaction with PGC remain unclear. In this study, we used an in vitro feeder-free culture system with chicken PGC as a model to explore the mechanisms by which RA induces the entry of PGC into meiosis. Results demonstrated that exogenous RA treatment altered the cell cycle distribution of PGC, significantly increasing the proportion of cells in the G1 phase and decreasing those in the G2 phase, suggesting that RA may promote the transition of PGC from proliferation to differentiation. Giemsa staining further revealed that chromosomes in a subset of RA-treated PGC exhibited meiotic characteristics. Through combined RNA-seq and ATAC-seq analyses, we identified that CYP26A1, a gene involved in RA degradation, was significantly upregulated in the RA-treated group, with enhanced accessibility in its chromatin regions. This finding suggests a robust mechanism for self-regulation of RA levels within PGC, indicating that CYP26A1 may play a pivotal role in the degradation of exogenous RA in chicken PGC. This study elucidated the effects of RA on chicken PGC and provided new insights into the role of RA in germ cell differentiation.
Keywords: ATAC-seq; RNA-seq; chicken primordial germ cell; meiosis; retinoic acid.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources

