Imaging the Ultrastructure of Isolated Peptidoglycan Sacculi from Rod-Shaped Helicobacter pylori J99 Cells by Atomic Force Microscopy
- PMID: 39795211
- PMCID: PMC11720842
- DOI: 10.3390/molecules30010155
Imaging the Ultrastructure of Isolated Peptidoglycan Sacculi from Rod-Shaped Helicobacter pylori J99 Cells by Atomic Force Microscopy
Abstract
Peptidoglycan is the basic structural polymer of the bacterial cell wall and maintains the shape and integrity of single cells. Despite years of research conducted on peptidoglycan's chemical composition, the microscopic elucidation of its nanoscopic architecture still needs to be addressed more thoroughly to advance knowledge on bacterial physiology. Apart from the model organism Escherichia coli, ultrastructural imaging data on the murein architecture of Gram-negative bacteria is mostly missing today. This study therefore intended to further our understanding of bacterial physiology by the isolation of peptidoglycan sacculi from the Gram-negative bacterium Helicobacter pylori J99 and the subsequent nanoscopic imaging of the murein network by Atomic Force Microscopy (AFM). With the ability to purify peptidoglycan sacculi from H. pylori J99 for AFM by a modified peptidoglycan isolation protocol, nanoscopic imaging of the murein network by intermittent-contact AFM in air and under liquid yielded ultrastructural insights into the H. pylori J99 cell wall architecture. High-resolution data acquisition on isolated peptidoglycan from H. pylori J99 by AFM under liquid was performed and revealed a molecular network similar to available data from E. coli. Subsequent enzymatic digestion of the isolated H. pylori J99 sacculi and analysis of the resulting fragments by +ESI-LCMS confirmed the presence of N-acetylglucosamine as an additional marker for successful peptidoglycan isolation. By comparison of the nanoscopic sacculus dimensions of H. pylori J99 to E. coli NU14, this study also identified specific differences in the sacculus morphology of both Gram-negative pathogenic bacteria.
Keywords: Atomic Force Microscopy; Helicobacter pylori J99; cell wall; peptidoglycan; ultrastructural imaging.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

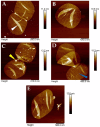


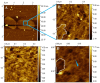
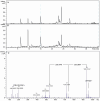
References
-
- Hooi J.K.Y., Lai W.Y., Ng W.K., Suen M.M.Y., Underwood F.E., Tanyingoh D., Malfertheiner P., Graham D.Y., Wong V.W.S., Wu J.C.Y., et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022. - DOI - PubMed
-
- Fung T.C., Vuong H.E., Luna C.D.G., Pronovost G.N., Aleksandrova A.A., Riley N.G., Vavilina A., McGinn J., Rendon T., Forrest L.R., et al. Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat. Microbiol. 2019;4:2064–2073. doi: 10.1038/s41564-019-0540-4. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

