Assessment of 3-Cyanobenzoic Acid as a Possible Herbicide Candidate: Effects on Maize Growth and Photosynthesis
- PMID: 39795261
- PMCID: PMC11722850
- DOI: 10.3390/plants14010001
Assessment of 3-Cyanobenzoic Acid as a Possible Herbicide Candidate: Effects on Maize Growth and Photosynthesis
Abstract
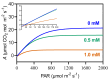
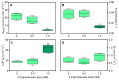
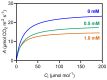
Chemical weed control is a significant agricultural concern, and reliance on a limited range of herbicide action modes has increased resistant weed species, many of which use C4 metabolism. As a result, the identification of novel herbicidal agents with low toxicity targeting C4 plants becomes imperative. An assessment was conducted on the impact of 3-cyanobenzoic acid on the growth and photosynthetic processes of maize (Zea mays), a representative C4 plant, cultivated hydroponically over 14 days. The results showed a significant reduction in plant growth and notable disruptions in gas exchange and chlorophyll a fluorescence due to the application of 3-cyanobenzoic acid, indicating compromised photosynthetic activity. Parameters such as the chlorophyll index, net assimilation (A), stomatal conductance (gs), intercellular CO2 concentration (Ci), maximum effective photochemical efficiency (Fv'/Fm'), photochemical quenching coefficient (qP), quantum yield of photosystem II photochemistry (ϕPSII), and electron transport rate through PSII (ETR) all decreased. The A/PAR curve revealed reductions in the maximum net assimilation rate (Amax) and apparent quantum yield (ϕ), alongside an increased light compensation point (LCP). Moreover, 3-cyanobenzoic acid significantly decreased the carboxylation rates of RuBisCo (Vcmax) and PEPCase (Vpmax), electron transport rate (J), and mesophilic conductance (gm). Overall, 3-cyanobenzoic acid induced substantial changes in plant growth, carboxylative processes, and photochemical activities. The treated plants also exhibited heightened susceptibility to intense light conditions, indicating a significant and potentially adverse impact on their physiological functions. These findings suggest that 3-cyanobenzoic acid or its analogs could be promising for future research targeting photosynthesis.
Keywords: chlorophyll a fluorescence; enzyme inhibitor; gas exchange; phenolics; phytotoxicity; pyruvate O-phosphate dikinase; weeds.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Talukder P., Sinha B., Biswas S., Ghosh A., Banerjee A., Paul S. A study on the prospect of converting C3 plants into C4 plants. Biocatal. Agric. Biotechnol. 2024;58:103191. doi: 10.1016/j.bcab.2024.103191. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

