Investigating the Allelopathic and Bioherbicidal Potential of Solidago altissima with a Focus on Chemical Signaling in Trifolium repens
- PMID: 39795356
- PMCID: PMC11723385
- DOI: 10.3390/plants14010096
Investigating the Allelopathic and Bioherbicidal Potential of Solidago altissima with a Focus on Chemical Signaling in Trifolium repens
Abstract
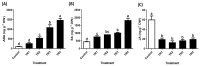
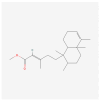
Invasive weed species exhibit both advantages, such as the potential for allelochemicals in bioherbicide development, and risks, including their threat to crop production. Therefore, this study aims to identify an allelochemical from Solidago altissima, an invasive weed species. The dose-dependent effects of S. altissima shoot and root extracts (SSE, SRE) on the signaling in the forage crop Trifolium repens and germination in various weed species (Echinochloa oryzicola, Cyperus microiria, Alopecurus aequalis, Portulaca oleracea, and Amaranthus retroflexus) were evaluated. The results showed that the T. repens seedlings treated with root extracts exhibited a significant decrease in plant height, dry weight, and chlorophyll content, along with an increase in H2O2 levels. Additionally, antioxidant activities, such as superoxide dismutase, catalase, and peroxidase enzyme activities, were significantly elevated in T. repens treated with SRE. Moreover, SRE treatment significantly inhibited the seed germination of all tested weed species in a concentration-dependent manner. Gas chromatography-mass spectrometry analysis of S. altissima root extract identified a high concentration of methyl kolavenate, a clerodane diterpene predicted to act as a phytotoxic agent. These findings highlight the potential of S. altissima for the development of crop-protective agents while emphasizing its potential risks in agriculture.
Keywords: ROS; antioxidants; methyl kolavenate; phytohormone; phytotoxicity; weeds.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
-
- Hussain M.I., Abideen Z., Danish S., Asghar M.A., Iqbal K. Integrated weed management for sustainable agriculture. Sustain. Agric. Rev. 2021;52:367–393.
-
- Etterson J.R., Delf D.E., Craig T.P., Ando Y., Ohgushi T. Parallel patterns of clinal variation in Solidago altissima in its native range in central USA and its invasive range in Japan. Botany. 2008;86:91–97. doi: 10.1139/B07-115. - DOI
-
- Maddox G.D., Cook R.E., Wimberger P.H., Gardescu S. Clone structure in four Solidago altissima (Asteraceae) populations: Rhizome connections within genotypes. Am. J. Bot. 1989;76:318–326. doi: 10.1002/j.1537-2197.1989.tb11315.x. - DOI
-
- Meyer A.H., Schmid B. Seed dynamics and seedling establishment in the invading perennial Solidago altissima under different experimental treatments. J. Ecol. 1999;87:28–41. doi: 10.1046/j.1365-2745.1999.00316.x. - DOI
-
- Cain M.L. Models of clonal growth in Solidago altissima. J. Ecol. 1990;78:27–46. doi: 10.2307/2261034. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

