Overexpression of AtruLEA1 from Acer truncatum Bunge Enhanced Arabidopsis Drought and Salt Tolerance by Improving ROS-Scavenging Capability
- PMID: 39795377
- PMCID: PMC11723042
- DOI: 10.3390/plants14010117
Overexpression of AtruLEA1 from Acer truncatum Bunge Enhanced Arabidopsis Drought and Salt Tolerance by Improving ROS-Scavenging Capability
Abstract
Late embryonic developmental abundant (LEA) genes play a crucial role in the response to abiotic stress and are important target genes for research on plant stress tolerance mechanisms. Acer truncatum Bunge is a promising candidate tree species for investigating the tolerance mechanism of woody plants against abiotic stress. In our previous study, AtruLEA1 was identified as being associated with seed drought tolerance. In this study, LEA1 was cloned from A. truncatum Bunge and functionally characterized. AtruLEA1 encodes an LEA protein and is located in the nucleus. Phylogenetic tree analysis revealed a recent affinity of the AtruLEA1 protein to AT3G15760.1. Overexpression of AtruLEA1 resulted in enhanced tolerance of Arabidopsis thaliana to drought and salt stress and heightened the ABA sensitivity. Compared to wild-type (WT) plants, plants with overexpressed AtruLEA1 exhibited increased activities of antioxidant enzymes under drought stress. Meanwhile, the ROS level of transgenic Arabidopsis was significantly less than that of the WT. Additionally, the stoma density and stoma openness of AtruLEA1 Arabidopsis were higher compared to those in the WT Arabidopsis under salt and drought stress conditions, which ensures that the biomass and relative water content of transgenic Arabidopsis are significantly better than those of the WT. These results indicated that AtruLEA1 was involved in salt and drought stress tolerances by maintaining ROS homeostasis, and its expression was positively regulated by abiotic stress. These results indicate a positive role of AtruLEA1 in drought and salt stress and provide theoretical evidence in the direction of cultivating resistant plants.
Keywords: ABA; Acer truncatum Bunge; LEA1; drought stress; salt stress.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures







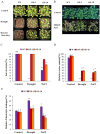

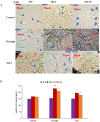
 and
and  means number of stomata in Arabidopsis,
means number of stomata in Arabidopsis,  means the normal stomata,
means the normal stomata,  means the stomata tended to close. All the images in the figure were acquired at 40× magnification under a microscope. Student’s t-test was used to determine significant differences (* p < 0.05 and ** p < 0.01).
means the stomata tended to close. All the images in the figure were acquired at 40× magnification under a microscope. Student’s t-test was used to determine significant differences (* p < 0.05 and ** p < 0.01).
Similar articles
-
PeGSTU58, a Glutathione S-Transferase from Populus euphratica, Enhances Salt and Drought Stress Tolerance in Transgenic Arabidopsis.Int J Mol Sci. 2023 May 27;24(11):9354. doi: 10.3390/ijms24119354. Int J Mol Sci. 2023. PMID: 37298311 Free PMC article.
-
PcNAC25, a NAC transcription factor of Pugionium cornutum(L.) Gaertn conferring enhanced drought and salt stress tolerances in Arabidopsis.Sci Rep. 2025 Jan 9;15(1):1501. doi: 10.1038/s41598-025-85615-w. Sci Rep. 2025. PMID: 39789053 Free PMC article.
-
A novel Cys2/His2 zinc finger protein gene from sweetpotato, IbZFP1, is involved in salt and drought tolerance in transgenic Arabidopsis.Planta. 2016 Mar;243(3):783-97. doi: 10.1007/s00425-015-2443-9. Epub 2015 Dec 21. Planta. 2016. PMID: 26691387
-
Birch (Betula platyphylla) BES/BZR transcription factor BpBZR1-6 improves salt tolerance in transgenic Arabidopsis thaliana.BMC Plant Biol. 2024 Nov 28;24(1):1136. doi: 10.1186/s12870-024-05738-6. BMC Plant Biol. 2024. PMID: 39604893 Free PMC article.
-
The Miscanthus NAC transcription factor MlNAC9 enhances abiotic stress tolerance in transgenic Arabidopsis.Gene. 2016 Jul 15;586(1):158-69. doi: 10.1016/j.gene.2016.04.028. Epub 2016 Apr 13. Gene. 2016. PMID: 27085481
Cited by
-
Metabolic Regulation and Saline-Alkali Stress Response in Novel Symbionts of Epichloë bromicola-Bromus inermis.Plants (Basel). 2025 Apr 1;14(7):1089. doi: 10.3390/plants14071089. Plants (Basel). 2025. PMID: 40219157 Free PMC article.
-
A Cold-Induced LEA3 Protein, DohD, Confers Cryoprotective Protection Against Low-Temperature Stress in Deinococcus radiodurans.Int J Mol Sci. 2025 Apr 9;26(8):3511. doi: 10.3390/ijms26083511. Int J Mol Sci. 2025. PMID: 40332004 Free PMC article.
References
-
- Candat A., Paszkiewicz G., Neveu M., Gautier R., Logan D.C., Avelange-Macherel M.H., Macherel D. The Ubiquitous Distribution of Late Embryogenesis Abundant Proteins across Cell Compartments in Arabidopsis Offers Tailored Protection against Abiotic Stress. Plant Cell. 2014;26:3148–3166. doi: 10.1105/tpc.114.127316. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

