Synthesis and Characterization of Copolymers with Fluorene-di-2-thienyl-2,1,3-benzothiadiazole Units for Application in Optoelectronic Devices
- PMID: 39795476
- PMCID: PMC11722960
- DOI: 10.3390/polym17010072
Synthesis and Characterization of Copolymers with Fluorene-di-2-thienyl-2,1,3-benzothiadiazole Units for Application in Optoelectronic Devices
Abstract
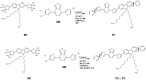
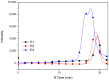
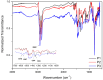
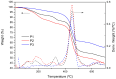
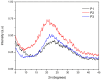
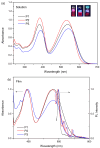
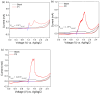
Conjugated donor-acceptor (D-A) copolymers are widely used in optoelectronic devices due to their influence on the resulting properties. This study focuses on the synthesis and characterization of the conjugated D-A copolymer constructed with fluorene and di-2-thienyl-2,1,3-benzothiadiazole units, resulting in Poly[2,7-(9,9-dioctyl-fluorene)-alt-5,5-(4,7-di(2-thienyl)-2,1,3-benzothiadiazole)] (PFDTBT). The synthesis associated with reaction times of 48 and 24 h, the latter incorporating the phase-transfer catalyst Aliquat 336, was investigated. The modified conditions produced copolymers with higher molar masses (Mw > 20,000 g/mol), improved thermal stability and red emission at 649 nm. Furthermore, the resulting D-A copolymers exhibited uniform morphology with low surface roughness (P2-Ra: 0.77 nm). These improved properties highlight the potential of D-A copolymers based on PFDTBT for various optoelectronic applications, including photovoltaics, light-emitting devices, transistors and biological markers in the form of quantum dots.
Keywords: D-A copolymers; electroluminescent polymers; red-emitting copolymer; synthesis of conjugated polymers.
Conflict of interest statement
The authors declare that this study received funding from the National Council for Scientific and Technological Development (CNPq, Brazil). The funder was not involved in study design, collection, analysis, interpretation of data, writing of this article, or the decision to submit it for publication.
Figures


References
-
- Pankow R.M., Thompson B.C. The development of conjugated polymers as the cornerstone of organic electronics. Polymer. 2020;207:122874. doi: 10.1016/j.polymer.2020.122874. - DOI
-
- Qiu Z., Hammer B.A.G., Müllen K. Conjugated polymers—Problems and promises. Prog. Polym. Sci. 2020;100:101179. doi: 10.1016/j.progpolymsci.2019.101179. - DOI
-
- Boivin L.P., Dupont W., Gendron D., Leclerc M. Biosourced Monomers: Toward Sustainable Conjugated Polymers for Organic Electronics. Macromol. Chem. Phys. 2023;224:2200378. doi: 10.1002/macp.202200378. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

