Leveraging Microneedles for Raised Scar Management
- PMID: 39795511
- PMCID: PMC11722619
- DOI: 10.3390/polym17010108
Leveraging Microneedles for Raised Scar Management
Abstract
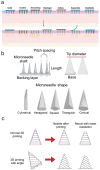
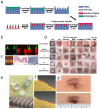
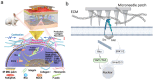
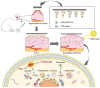
Disruption of the molecular pathways during physiological wound healing can lead to raised scar formation, characterized by rigid, thick scar tissue with associated symptoms of pain and pruritus. A key mechanical factor in raised scar development is excessive tension at the wound site. Recently, microneedles (MNs) have emerged as promising tools for scar management as they engage with scar tissue and provide them with mechanical off-loading from both internal and external sources. This review explores the mechanisms by which physical intervention of drug-free MNs alleviates mechanical tension on fibroblasts within scar tissue, thereby promoting tissue remodeling and reducing scar severity. Additionally, the role of MNs as an efficient cargo delivery system for the controlled and sustained release of a wide range of therapeutic agents into scar tissue is highlighted. By penetrating scar tissue, MNs facilitate controlled and sustained localized drug administration to modulate inflammation and fibroblastic cell growth. Finally, the remaining challenges and the future perspective of the field have been highlighted.
Keywords: drug delivery; hydrogels; hypertrophic scar; keloids; microneedle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

