4-Pyridone-3-carboxamide-1-β-D-ribonucleoside Reduces Cyclophosphamide Effects and Induces Endothelial Inflammation in Murine Breast Cancer Model
- PMID: 39795893
- PMCID: PMC11719935
- DOI: 10.3390/ijms26010035
4-Pyridone-3-carboxamide-1-β-D-ribonucleoside Reduces Cyclophosphamide Effects and Induces Endothelial Inflammation in Murine Breast Cancer Model
Abstract
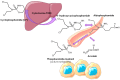
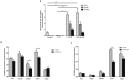
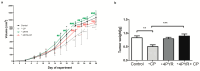
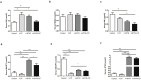
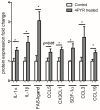
4-pyridone-3-carboxamide-1-β-D-ribonucleoside (4PYR) is a nicotinamide derivative, considered a new oncometabolite. 4PYR formation induced a cytotoxic effect on the endothelium. Elevated blood 4PYR concentration was observed in patients with cancer. Still, little is known about the metabolic and functional effects of 4PYR in this pathology. The study aimed to investigate whether this toxic accumulation of 4PYR may affect the activity of anticancer therapy with cyclophosphamide in the orthotropic model of breast cancer. Female Balb/c mice were injected with 4T1 breast cancer cells and assigned into three groups: treated with PBS (Control), cyclophosphamide-treated (+CP), 4PYR-treated (+4PYR), and mice treated with both 4PYR and CP(+4PYR+CP) for 28 days. Afterward, blood and serum samples, liver, muscle, spleen, heart, lungs, aortas, and tumor tissue were collected for analysis of concentrations of nucleotides, nicotinamide metabolites, and 4PYR with its metabolites, as well as the liver level of cytochrome P450 enzymes. 4PYR treatment caused elevation of blood 4PYR, its monophosphate and a nicotinamide adenine dinucleotide (NAD+) analog-4PYRAD. Blood 4PYRAD concentration in the +4PYR+CP was reduced in comparison to +4PYR. Tumor growth and final tumor mass were significantly decreased in +CP and did not differ in +4PYR in comparison to Control. However, we observed a substantial increase in these parameters in +4PYR+CP as compared to +CP. The extracellular adenosine deamination rate was measured to assess vascular inflammation, and it was higher in +4PYR than the Control. Treatment with 4PYR and CP caused the highest vascular ATP hydrolysis and adenosine deamination rate. 4PYR administration caused significant elevation of CYP2C9 and reduction in CYP3A4 liver concentrations in both +4PYR and +4PYR+CP as compared to Control and +CP. In additional experiments, we compared healthy mice without cancer, treated with 4PYR (4PYR w/o cancer) and PBS (Control w/o cancer), where 4PYR treatment caused an increase in the serum proinflammatory cytokine expression as compared to Control w/o cancer. 4PYR accumulation in the blood interferes with cyclophosphamide anticancer activity and induces a pro-inflammatory shift of endothelial extracellular enzymes, probably by affecting its metabolism by cytochrome P450 enzymes. This observation may have crucial implications for the activity of various anticancer drugs metabolized by cytochrome P450.
Keywords: breast cancer; cyclophosphamide; nicotinamide metabolism.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
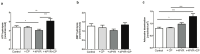
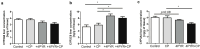
Similar articles
-
Influence of 4-pyridone-3-carboxamide-1Β-D-ribonucleoside (4PYR) on activities of extracellular enzymes in endothelial human cells.Nucleosides Nucleotides Nucleic Acids. 2016 Dec;35(10-12):732-736. doi: 10.1080/15257770.2016.1174263. Nucleosides Nucleotides Nucleic Acids. 2016. PMID: 27906624
-
An unusual nicotinamide derivative, 4-pyridone-3-carboxamide ribonucleoside (4PYR), is a novel endothelial toxin and oncometabolite.Exp Mol Med. 2021 Sep;53(9):1402-1412. doi: 10.1038/s12276-021-00669-w. Epub 2021 Sep 27. Exp Mol Med. 2021. PMID: 34580423 Free PMC article.
-
4-Pyridone-3-carboxamide-1β-D-ribonucleoside metabolism in endothelial cells and its impact on cellular energetic balance.Nucleosides Nucleotides Nucleic Acids. 2014;33(4-6):338-41. doi: 10.1080/15257770.2014.889303. Nucleosides Nucleotides Nucleic Acids. 2014. PMID: 24940690
-
Endothelial toxicity of unusual nucleotide metabolites.Pharmacol Rep. 2015 Aug;67(4):818-22. doi: 10.1016/j.pharep.2015.03.020. Epub 2015 Apr 15. Pharmacol Rep. 2015. PMID: 26321286 Review.
-
Cellular toxicity of nicotinamide metabolites.J Ren Nutr. 2012 Jan;22(1):95-7. doi: 10.1053/j.jrn.2011.10.033. J Ren Nutr. 2012. PMID: 22200423 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

