Single Disulfide Bond in Host Defense Thanatin Analog Peptides: Antimicrobial Activity, Atomic-Resolution Structures and Target Interactions
- PMID: 39795909
- PMCID: PMC11720011
- DOI: 10.3390/ijms26010051
Single Disulfide Bond in Host Defense Thanatin Analog Peptides: Antimicrobial Activity, Atomic-Resolution Structures and Target Interactions
Abstract
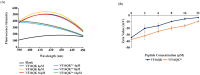
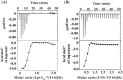
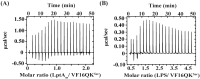
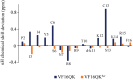
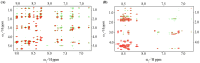
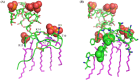
Host defense antimicrobial peptides (AMPs) are promising lead molecules with which to develop antibiotics against drug-resistant bacterial pathogens. Thanatin, an inducible antimicrobial peptide involved in the host defense of Podisus maculiventris insects, is gaining considerable attention in the generation of novel classes of antibiotics. Thanatin or thanatin-based analog peptides are extremely potent in killing bacterial pathogens in the Enterobacteriaceae family, including drug-resistant strains of Escherichia coli and Klebsiella pneumoniae. A single disulfide bond that covalently links two anti-parallel β-strands in thanatin could be pivotal to its selective antibacterial activity and mode of action. However, potential correlations of the disulfide covalent bond with structure, activity and target binding in thanatin peptides are currently unclear to. Here, we examined a 16-residue designed thanatin peptide, namely disulfide-bonded VF16QK, and its Cys to Ser substituted variant, VF16QKSer, to delineate their structure-activity relationships. Bacterial growth inhibitory activity was only detected for the disulfide-bonded VF16QK peptide. Mechanistically, both peptides vastly differ in their bacterial cell permeabilizations, atomic-resolution structures, interactions with the LPS-outer membrane and target periplasmic protein LptAm binding. In particular, analysis of the 3-D structures of the two peptides revealed an altered folded conformation for the VF16QKSer peptide that was correlated with diminished LPS-outer membrane permeabilization and target interactions. Analysis of docked complexes of LPS-thanatin peptides indicated potential structural requirements and conformational adaptation for antimicrobial activity. Collectively, these observations contrast with those for the disulfide-bonded β-hairpin antimicrobial protegrin and tachyplesin peptides, where disulfide bonds are dispensable for activity. We surmise that the atomistic structures and associated molecular interactions presented in this work can be utilized to design novel thanatin-based antibiotics.
Keywords: LPS; LptA; LptAm; NMR; host defense antimicrobial peptide; thanatin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- O’Neill J. The Review on Antimicrobial Resistance (AMR) Government of the United Kingdom; London, UK: 2016. Tackling drug-resistant infections globally: Final report and recommendations.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

