Immune Checkpoint Inhibitor-Associated Cutaneous Adverse Events: Mechanisms of Occurrence
- PMID: 39795946
- PMCID: PMC11719825
- DOI: 10.3390/ijms26010088
Immune Checkpoint Inhibitor-Associated Cutaneous Adverse Events: Mechanisms of Occurrence
Abstract
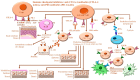
Immunotherapy, particularly that based on blocking checkpoint proteins in many tumors, including melanoma, Merkel cell carcinoma, non-small cell lung cancer (NSCLC), triple-negative breast (TNB cancer), renal cancer, and gastrointestinal and endometrial neoplasms, is a therapeutic alternative to chemotherapy. Immune checkpoint inhibitor (ICI)-based therapies have the potential to target different pathways leading to the destruction of cancer cells. Although ICIs are an effective treatment strategy for patients with highly immune-infiltrated cancers, the development of different adverse effects including cutaneous adverse effects during and after the treatment with ICIs is common. ICI-associated cutaneous adverse effects include mostly inflammatory and bullous dermatoses, as well as severe cutaneous side reactions such as rash or inflammatory dermatitis encompassing erythema multiforme; lichenoid, eczematous, psoriasiform, and morbilliform lesions; and palmoplantar erythrodysesthesia. The development of immunotherapy-related adverse effects is a consequence of ICIs' unique molecular action that is mainly mediated by the activation of cytotoxic CD4+/CD8+ T cells. ICI-associated cutaneous disorders are the most prevalent effects induced in response to anti-programmed cell death 1 (PD-1), anti-cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), and anti-programmed cell death ligand 1 (PD-L1) agents. Herein, we will elucidate the mechanisms regulating the occurrence of cutaneous adverse effects following treatment with ICIs.
Keywords: CAR T cells; CTLA-4; ICIs; PD-1.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
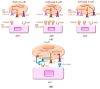

 means negative signal; while green arrow:
means negative signal; while green arrow:  means weak or positive siganal.
means weak or positive siganal.
 means negative signal; while green arrow:
means negative signal; while green arrow:  means weak or positive siganal.
means weak or positive siganal.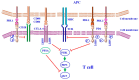
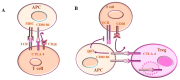
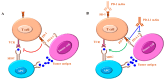
References
-
- Chang C.-Y., Park H., Malone D.C., Wang C.-Y., Wilson D.L., Yeh Y.-M., Van Boemmel-Wegmann S., Lo-Ciganic W.-H. Immune Checkpoint Inhibitors and Immune-Related Adverse Events in Patients With Advanced Melanoma. JAMA Netw. Open. 2020;3:e201611. doi: 10.1001/jamanetworkopen.2020.1611. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

