Direct Vascular Effects of Angiotensin II (A Systematic Short Review)
- PMID: 39795971
- PMCID: PMC11719566
- DOI: 10.3390/ijms26010113
Direct Vascular Effects of Angiotensin II (A Systematic Short Review)
Abstract
The octapeptide angiotensin II (Ang II) is a circulating hormone as well as a locally formed agonist synthesized by the angiotensin-converting enzyme (ACE) of endothelial cells. It forms a powerful mechanism to control the amount and pressure of body fluids. All main effects are directed to save body salt and water and ensure blood pressure under basic conditions and in emergencies. All blood vessels respond to stimulation by Ang II; the immediate response is smooth muscle contraction, increasing vascular resistance, and elevating blood pressure. Such effects are conveyed by type 1 angiotensin receptors (AT1Rs) located in the plasma membrane of both endothelial and vascular smooth muscle cells. AT1Rs are heterotrimeric G protein-coupled receptors (GPCRs), but their signal pathways are much more complicated than other GPCRs. In addition to Gq/11, the G12/13, JAK/STAT, Jnk, MAPK, and ERK 1/2, and arrestin-dependent and -independent pathways are activated because of the promiscuous attachment of different signal proteins to the intracellular G protein binding site and to the intracellular C terminal loop. Substantial changes in protein expression follow, including the intracellular inflammation signal protein NF-κB, endothelial contact proteins, cytokines, matrix metalloproteinases (MMPs), and type I protocollagen, eliciting the inflammatory transformation of endothelial and vascular smooth muscle cells and fibrosis. Ang II is an important contributor to vascular pathologies in hypertensive, atherosclerotic, and aneurysmal vascular wall remodeling. Such direct vascular effects are reviewed. In addition to reducing blood pressure, AT1R antagonists and ACE inhibitors have a beneficial effect on the vascular wall by inhibiting pathological wall remodeling.
Keywords: angiotensin II; angiotensin receptors; renin-angiotensin system; vascular effects; vascular remodeling.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
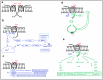


References
-
- Nadasy G.L., Balla A., Szekeres M. From living in saltwater to a scarcity of salt and water, and then an overabundance of salt—The biological roller-coaster to which the renin-angiotensin system has had to adapt: An editorial. Biomedicines. 2023;11:3004. doi: 10.3390/biomedicines11113004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- no grant source/Semmelweis University
- e1b27034e34a656b 3e0d9d5ea8e1668b 1801b9bcd56bdc35 f444fdb2e1d0609c 7e4a83222051089a f32c908578ba9a95 88edd9ef9f159419 87d249260f493835 662b86617b4e4321 55880db6707ca982 f036b612e00fdd0c 6f0a8d9e8f8cb302 51643a80d769cc4f 5d59940746a2e9e3 eb23c5832160d4de/MDPI Vouchers
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

