Key Interleukins in Inflammatory Bowel Disease-A Review of Recent Studies
- PMID: 39795980
- PMCID: PMC11719876
- DOI: 10.3390/ijms26010121
Key Interleukins in Inflammatory Bowel Disease-A Review of Recent Studies
Abstract
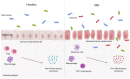
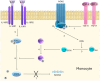
Inflammatory bowel disease (IBD) is an immune disorder of the gastrointestinal tract with a complex aetiopathogenesis, whose development is influenced by many factors. The prevalence of IBD is increasing worldwide, in both industrialized and developing countries, making IBD a global health problem that seriously affects quality of life. In 2019, there were approximately 4.9 million cases of IBD worldwide. Such a large number of patients entails significant healthcare costs. In the treatment of patients with IBD, the current therapeutic target is mucosal healing, as intestinal inflammation often persists despite resolution of abdominal symptoms. Treatment strategies include amino salicylates, corticosteroids, immunosuppressants, and biologic therapies that focus on reducing intestinal mucosal inflammation, inducing and prolonging disease remission, and treating complications. The American College of Gastroenterology (ACG) guidelines also indicate that nutritional therapies may be considered in addition to other therapies. However, current therapeutic approaches are not fully effective and are associated with various limitations, such as drug resistance, variable efficacy, and side effects. As the chronic inflammation that accompanies IBD is characterized by infiltration of a variety of immune cells and increased expression of a number of pro-inflammatory cytokines, including IL-6, TNF-α, IL-12, IL-23 and IFN-γ, new therapeutic approaches are mainly targeting immune pathways. Interleukins are one of the molecular targets in IBD therapy. Interleukins and related cytokines serve as a means of communication for innate and adaptive immune cells, as well as nonimmune cells and tissues. These cytokines play an important role in the pathogenesis and course of IBD, making them promising targets for current and future therapies. In our work, we review scientific studies published between January 2022 and November 2024 describing the most important interleukins involved in the pathogenesis of IBD. Some of the papers present new data on the precise role that individual interleukins play in IBD. New clinical data have also been provided, particularly on blocking interleukin 23 and interleukin 1beta. In addition, several new approaches to the use of different interleukins in the treatment of IBD have been described in recent years.
Keywords: Crohn’s disease; inflammatory bowel disease; interleukin 10; interleukin 12; interleukin 17; interleukin 1β; interleukin 22; interleukin 23; interleukin 33; interleukin 6; interleukins; recent studies; ulcerative colitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Alperen C.C., Soydas B., Serin E., Erbayrak M., Savas N.A., Unler G.K., Meral C.E., Toprak U., Boyacioglu A.S., Dagli U. Role of Environmental Risk Factors in the Etiology of Inflammatory Bowel Diseases: A Multicenter Study. Dig. Dis. Sci. 2024;69:2927–2936. doi: 10.1007/s10620-024-08491-w. - DOI - PubMed
-
- Chu X., Chen X., Zhang H., Wang Y., Guo H., Chen Y., Liu X., Zhu Z., He Y., Ding X., et al. Association of diet and outdoor time with inflammatory bowel disease: A multicenter case-control study using propensity matching analysis in China. Front. Public. Health. 2024;12:1368401. doi: 10.3389/fpubh.2024.1368401. - DOI - PMC - PubMed
-
- Furuya Y., Fukai K., Nakazawa S., Kojimahara N., Hoshi K., Toyota A., Tatemichi M. Occupational physical activity differentially affects the risk for developing later-onset Crohn’s disease and ulcerative colitis among middle-aged and older populations. Scand. J. Gastroenterol. 2022;57:206–213. doi: 10.1080/00365521.2021.1999495. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

