Recent Advances in the Search for Effective Anti-Alzheimer's Drugs
- PMID: 39796014
- PMCID: PMC11720639
- DOI: 10.3390/ijms26010157
Recent Advances in the Search for Effective Anti-Alzheimer's Drugs
Abstract
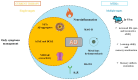
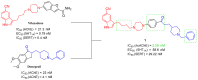
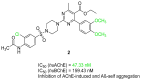
Alzheimer's disease, the most common form of dementia, is characterized by the deposition of amyloid plaques and neurofibrillary tangles in the brain, leading to the loss of neurons and a decline in a person's memory and cognitive function. As a multifactorial disease, Alzheimer's involves multiple pathogenic mechanisms, making its treatment particularly challenging. Current drugs approved for the treatment of Alzheimer's disease only alleviate symptoms but cannot stop the progression. Moreover, these drugs typically target a single pathogenic mechanism, leaving other contributing factors unaddressed. Recent advancements in drug design have led to the development of multi-target-directed ligands (MTDLs), which have gained popularity for their ability to simultaneously target multiple pathogenic mechanisms. This paper focuses on analyzing the activity, mechanism of action, and binding properties of the anti-Alzheimer's MTDLs developed between 2020 and 2024.
Keywords: AChE inhibitors; Alzheimer’s disease; BChE inhibitors; donepezil; multi-target-directed ligands; neurodegenerative disorders; tacrine; β-amyloid aggregation inhibitors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
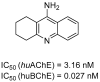
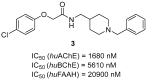


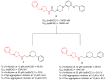
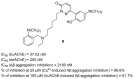
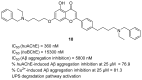
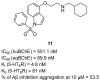
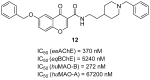

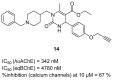
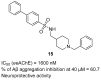

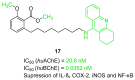
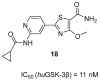
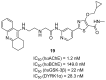
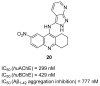

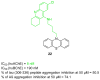
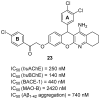
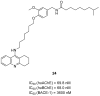
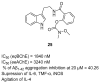
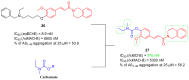
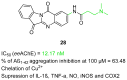
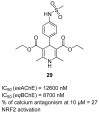
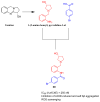
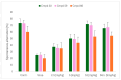
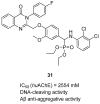
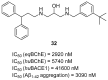
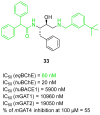
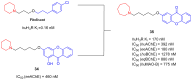
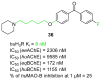
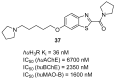
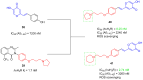



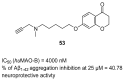
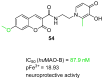
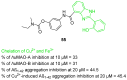

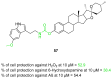
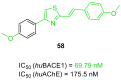
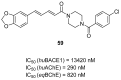
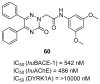
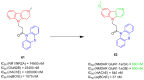
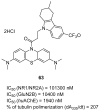

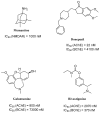
References
-
- World Health Organisation Dementia. 2023. [(accessed on 30 December 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

