Atrial Fibrosis in Atrial Fibrillation: Mechanistic Insights, Diagnostic Challenges, and Emerging Therapeutic Targets
- PMID: 39796066
- PMCID: PMC11720255
- DOI: 10.3390/ijms26010209
Atrial Fibrosis in Atrial Fibrillation: Mechanistic Insights, Diagnostic Challenges, and Emerging Therapeutic Targets
Abstract
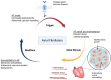
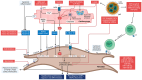
Atrial fibrosis is a hallmark of atrial cardiomyopathy and plays a pivotal role in the pathogenesis of atrial fibrillation (AF), contributing to its onset and progression. The mechanisms underlying atrial fibrosis are multifaceted, involving stretch-induced fibroblast activation, oxidative stress, inflammation, and coagulation pathways. Variations in fibrosis types-reactive and replacement fibrosis-are influenced by patient-specific factors such as age, sex, and comorbidities, complicating therapeutic approaches. The heterogeneity of fibrosis leads to distinct electrophysiological abnormalities that promote AF via reentrant activity and enhanced automaticity mechanisms. Despite advancements in imaging, such as late gadolinium enhancement CMR and electroanatomical mapping, challenges in accurately quantifying fibrosis persist. Emerging therapeutic strategies include antifibrotic agents targeting the renin-angiotensin-aldosterone system, novel pathways like TGF-β signaling, and cardio-metabolic drugs like SGLT2 inhibitors and GLP-1 receptor agonists. Innovative interventions, including microRNA modulation and lipid nanoparticle-based therapies, show promise but require validation. Knowledge gaps remain in correlating clinical outcomes with fibrosis patterns and optimizing diagnostic tools. Future research should focus on precise phenotyping, integrating advanced imaging with molecular biomarkers, and conducting robust trials to evaluate antifibrotic therapies' efficacy in reducing AF burden and related complications.
Keywords: GLP1 receptor agonists; SGLT2 inhibitors; arrhythmogenic mechanism; atrial fibrillation; atrial fibrosis; inflammation; protease-activated receptor inhibitors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Goette A., Corradi D., Dobrev D., Aguinaga L., Cabrera J.-A., Chugh S.S., de Groot J.R., Soulat-Dufour L., Fenelon G., Hatem S.N., et al. Atrial Cardiomyopathy Revisited-Evolution of a Concept: A Clinical Consensus Statement of the European Heart Rhythm Association (EHRA) of the ESC, the Heart Rhythm Society (HRS), the Asian Pacific Heart Rhythm Society (APHRS), and the Latin American Hear. EP Eur. 2024;26:euae204. doi: 10.1093/europace/euae204. - DOI - PMC - PubMed
-
- Miyauchi S., Tokuyama T., Takahashi S., Hiyama T., Okubo Y., Okamura S., Miyamoto S., Oguri N., Takasaki T., Katayama K., et al. Relationship Between Fibrosis, Endocardial Endothelial Damage, and Thrombosis of Left Atrial Appendage in Atrial Fibrillation. JACC Clin. Electrophysiol. 2023;9:1158–1168. doi: 10.1016/j.jacep.2023.01.029. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

