Prolonged Hypoxia in Rat Living Myocardial Slices Affects Function, Expression, and Structure
- PMID: 39796086
- PMCID: PMC11720517
- DOI: 10.3390/ijms26010218
Prolonged Hypoxia in Rat Living Myocardial Slices Affects Function, Expression, and Structure
Abstract
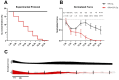
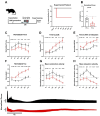
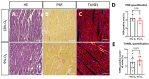
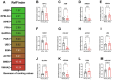
Ischemic heart disease is the leading cause of death worldwide. Reduced oxygen supply and myocardial hypoxia lead to tissue damage and impairment of the heart function. To the best of our knowledge, the primary functional effects of hypoxia in the multicellular model of living myocardial slices (LMSs) have not been investigated so far. In this study, we analyzed force generation, ultrastructure, gene expression, and proteome changes in rat LMS after 24 h of ex vivo culture in normal and reduced levels of oxygen (O2). We observed a significant reduction in absolute force and a slowdown of force kinetics as well as an increase in cardiomyocyte apoptosis and myofibrillar and mitochondrial damage, as well as transcriptomic changes. Proteome analysis revealed the deregulation of proteins involved in metabolic processes, hypoxic response, and neutralizing of reactive oxygen species. Our results indicate that hypoxia induces substantial primary changes in heart tissue, which are independent of perfusion and immune responses. Our new LMS model could serve as a screening system for drug development and new mechanistic insights.
Keywords: force generation; gene expression; hypoxia; proteome; rat living myocardial slices; tissue model.
Conflict of interest statement
T.T. filed and licensed patents in the field of RNA-based therapeutics. T.T. is the founder and CSO/CMO of Cardior Pharmaceuticals Gmbh, a wholly-owned subsidiary of Novo Nordisk A/S Europe (outside of this MS). All other authors declare no conflicts of interest.
Figures

References
-
- Khan M.A., Hashim M.J., Mustafa H., Baniyas M.Y., Al Suwaidi S.K.B.M., AlKatheeri R., Alblooshi F.M.K., Almatrooshi M.E.A.H., Alzaabi M.E.H., Al Darmaki R.S., et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus. 2020;12:e9349. doi: 10.7759/cureus.9349. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

