Systematic Evaluation of Extracellular Coating Matrix on the Differentiation of Human-Induced Pluripotent Stem Cells to Cortical Neurons
- PMID: 39796088
- PMCID: PMC11720352
- DOI: 10.3390/ijms26010230
Systematic Evaluation of Extracellular Coating Matrix on the Differentiation of Human-Induced Pluripotent Stem Cells to Cortical Neurons
Abstract
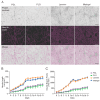
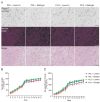
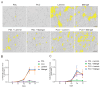
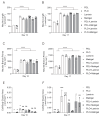
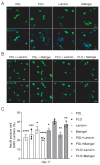
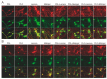
Induced pluripotent stem cell (iPSC)-derived neurons (iNs) have been widely used as models of neurodevelopment and neurodegenerative diseases. Coating cell culture vessels with extracellular matrixes (ECMs) gives structural support and facilitates cell communication and differentiation, ultimately enhances neuronal functions. However, the relevance of different ECMs to the natural environment and their impact on neuronal differentiation have not been fully characterized. In this study, we report the use of four commonly used extracellular matrixes, poly-D-lysine (PDL), poly-L-ornithine (PLO), Laminin and Matrigel, which we applied to compare the single-coating and double-coating conditions on iNs differentiation and maturation. Using the IncuCyte live-cell imaging system, we found that iNs cultured on single Matrigel- and Laminin-coated vessels have significantly higher density of neurite outgrowth and branch points than PLO or PDL but produce abnormal highly straight neurite outgrowth and larger cell body clumps. All the four double-coating conditions significantly reduced the clumping of neurons, in which the combination of PDL+Matrigel also enhanced neuronal purity. Double coating with PDL+Matrigel also tended to improve dendritic and axonal development and the distribution of pre and postsynaptic markers. These results demonstrate that the extracellular matrix contributes to the differentiation of cultured neurons and that double coating with PDL+Matrigel gives the best outcomes. Our study indicates that neuronal differentiation and maturation can be manipulated, to a certain extent, by adjusting the ECM recipe, and provides important technical guidance for the use of the ECM in neurological studies.
Keywords: induced pluripotent stem cells (iPSCs); live-cell imaging; morphology; neurite outgrowth; neuronal differentiation; synaptic markers.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Bayati A., Ayoubi R., Aguila A., Zorca C.E., Deyab G., Han C., Recinto S.J., Nguyen-Renou E., Rocha C., Maussion G., et al. Modeling Parkinson’s disease pathology in human dopaminergic neurons by sequential exposure to α-synuclein fibrils and proinflammatory cytokines. Nat. Neurosci. 2024;27:2401–2416. doi: 10.1038/s41593-024-01775-4. - DOI - PubMed
-
- Tsitkov S., Valentine K., Kozareva V., Donde A., Frank A., Lei S., EVan Eyk J., Finkbeiner S., Rothstein J.D., Thompson L.M., et al. Disease related changes in ATAC-seq of iPSC-derived motor neuron lines from ALS patients and controls. Nat. Commun. 2024;15:3606. doi: 10.1038/s41467-024-47758-8. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 32100800/National Natural Science Foundation of China
- 32471010/National Natural Science Foundation of China
- JCYJ20220818100802005/Shenzhen Science and Technology Innovation Program
- KQTD20210811090117032/Shenzhen Science and Technology Innovation Program
- ZDSYS20220304163558001/Shenzhen Key Laboratory of Neuroimmunomodulation for Neurological Diseases
LinkOut - more resources
Full Text Sources

