Dynamic Changes in Circulating Tumor DNA During Immunotherapy for Head and Neck Cancer: SHIZUKU-HN Study
- PMID: 39796090
- PMCID: PMC11719933
- DOI: 10.3390/ijms26010235
Dynamic Changes in Circulating Tumor DNA During Immunotherapy for Head and Neck Cancer: SHIZUKU-HN Study
Abstract
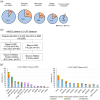
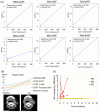
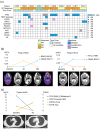
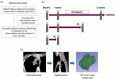
Immune checkpoint inhibitors (ICIs) are effective in treating recurrent/metastatic head and neck squamous cell carcinoma (HNSCC), but only 20% of patients achieve durable responses. This study evaluated circulating tumor DNA (ctDNA) as a real-time biomarker for monitoring treatment response in HNSCC. The SHIZUKU-HN study prospectively collected and analyzed serial plasma samples (n = 27) from HNSCC patients undergoing ICIs, using Guardant360 to assess ctDNA variant allele frequency (VAF) and genetic mutations. Tumor volumes were quantified using 3D reconstruction of CT scans, and data from Japan's C-CAT database (n = 2255) provided insights into ctDNA testing in HNSCC. C-CAT data showed that ctDNA testing was underutilized, performed in only 7% of head and neck cancer cases. In SHIZUKU-HN, mean VAF significantly correlated with tumor volume (Spearman's ρ = 0.70, p = 0.001), often preceding radiographic progression. BRAF and APC mutations disappeared in partial responders, while GNAS mutations varied. EGFR and PIK3CA amplifications, detectable via ctDNA but missed in tissue biopsies, indicated emerging resistance mechanisms. The SHIZUKU-HN study demonstrates the potential of ctDNA as a dynamic biomarker in HNSCC, offering early insights into treatment efficacy and informing personalized ICI therapy.
Keywords: Center for Cancer Genomics and Advanced Therapeutics (C-CAT); circulating tumor DNA (ctDNA); dynamic monitoring; head and neck squamous cell carcinoma (HNSCC); immune checkpoint inhibitor (ICI); liquid biopsy; variant allele frequency (VAF).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Burtness B., Harrington K.J., Greil R., Soulières D., Tahara M., de Castro G., Jr., Psyrri A., Basté N., Neupane P., Bratland Å., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet. 2019;394:1915–1928. doi: 10.1016/S0140-6736(19)32591-7. - DOI - PubMed
-
- Noji R., Tohyama K., Kugimoto T., Kuroshima T., Hirai H., Tomioka H., Michi Y., Tasaki A., Ohno K., Ariizumi Y., et al. Comprehensive Genomic Profiling Reveals Clinical Associations in Response to Immune Therapy in Head and Neck Cancer. Cancers. 2022;14:3476. doi: 10.3390/cancers14143476. - DOI - PMC - PubMed
-
- Naito T., Noji R., Kugimoto T., Kuroshima T., Tomioka H., Fujiwara S., Suenaga M., Harada H., Kano Y. The Efficacy of Immunotherapy and Clinical Utility of Comprehensive Genomic Profiling in Adenoid Cystic Carcinoma of Head and Neck. Medicina. 2023;59:2111. doi: 10.3390/medicina59122111. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

