Clove Essential Oil as a Source of Antitumoral Compounds Capable of Crossing the Blood-Brain Barrier: A Focus on the Effects of β-Caryophyllene and Eugenol in a Glioblastoma Cell Line
- PMID: 39796096
- PMCID: PMC11720353
- DOI: 10.3390/ijms26010238
Clove Essential Oil as a Source of Antitumoral Compounds Capable of Crossing the Blood-Brain Barrier: A Focus on the Effects of β-Caryophyllene and Eugenol in a Glioblastoma Cell Line
Abstract
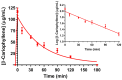
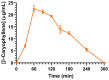
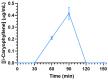
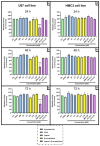
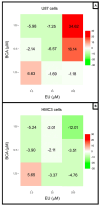
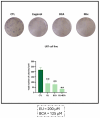
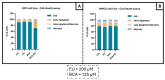
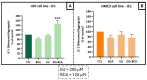
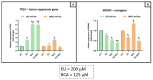
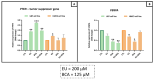
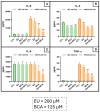
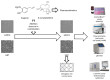
This study aimed to investigate β-Caryophyllene (BCA) pharmacokinetics as well as the potential antitumor activity and mechanism of action of BCA and eugenol (EU), alone or in combination, in U87 glioblastoma (GB) cells. The BCA pharmacokinetic was studied by evaluating its concentration profiles in rat blood and cerebrospinal fluid after oral and intravenous administration. EU and BCA antitumor mechanisms were assessed by comparing their effects in U87 GB cells and non-tumoral HMC3 cells. Cell death, cell cycle regulation and mitochondrial membrane potential (MMP) were evaluated using flow cytometry. mRNA levels of target genes were evaluated by qPCR. Secreted cytokines were measured by Luminex®. BCA, as well as EU, permeates the brain. EU and BCA affected the viability and proliferation of U87 cells (up to 50%, p < 0.001) but not HMC3 cells and showed a synergistic effect. BCA and EU induced G0/G1 cell cycle arrest, increasing apoptosis/necrosis. EU and BCA induced the downregulation of mRNAs encoding for key proteins involved in GB angiogenesis (VEGFA decreased op to 60%, p < 0.01), proliferation and progression, and showed anti-inflammatory activity (IL-4 significantly decreased, p < 0.001). EU and BCA demonstrated strong and multitarget antitumor activity in U87 cells. Our results provide a strong rationale for the further evaluation of EU and BCA as possible therapeutic molecules in GB management.
Keywords: antitumor activity; essential oils; eugenol; glioblastoma; pharmacokinetics; β-Caryophyllene.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncology. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. - DOI - PMC - PubMed
-
- Waqar M., Roncaroli F., Lehrer E.J., Palmer J.D., Villanueva-Meyer J., Braunstein S., Hall E., Aznar M., De Witt Hamer P.C., D’Urso P.I., et al. Rapid Early Progression (REP) of Glioblastoma Is an Independent Negative Prognostic Factor: Results from a Systematic Review and Meta-Analysis. Neuro-Oncol. Adv. 2022;4:vdac075. doi: 10.1093/noajnl/vdac075. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

