Amelioration Effect of Lactobacillus kefiranofaciens ZW3 on Ovalbumin-Induced Allergic Symptoms in BALB/c Mice
- PMID: 39796306
- PMCID: PMC11720023
- DOI: 10.3390/foods14010016
Amelioration Effect of Lactobacillus kefiranofaciens ZW3 on Ovalbumin-Induced Allergic Symptoms in BALB/c Mice
Abstract
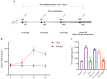
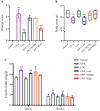
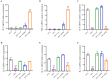
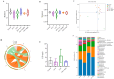
Previous studies have shown that supplementation with specific probiotics can be used to alleviate allergy symptoms. The purpose of this study was to evaluate the anti-allergic effects of Lactobacillus kefiranofaciens ZW3 (ZW3) in ovalbumin (OVA)-induced allergic mice. The mice were divided into six groups: the food allergy group, positive group (Lactobacillus rhamnosus GG), low-dose ZW3 group, middle-dose ZW3 group, high-dose ZW3 group, and the control group involving healthy mice. BALB/c mice were intraperitoneally injected with OVA/complete Freund's adjuvant (CFA) for allergy sensitization. Probiotics were administered orally once every two days in the probiotic-treated groups. The allergic score, serum OVA-sIgE, body mass, thymus, and spleen indexes were detected on day 22, and the relative mRNA expression of inflammatory cytokines was detected via RT-qPCR. The results suggest that the body weight and thymus index returned to normal levels; allergy scores, serum OVA-sIgE, IL-4, IL-5, and IL-10 expression decreased; and IFN-γ and IL-2 increased significantly in the ZW3 group compared with the allergy group. Furthermore, ZW3 decreased Muribaculaceae and Ruminococcaceae abundance and increased Lachnospiraceae abundance in the intestinal flora. In summary, ZW3 induced anti-allergic effects by increasing Th1 cytokines and decreasing Th2 cytokines, which can remarkably ameliorate the symptoms of an ovalbumin-induced food allergy.
Keywords: Lactobacillus kefiranofaciens; Th1/Th2 balance; allergic symptoms; intestinal flora.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
[Lactobacillus rhamnosus GG improves symptoms and its mechnism in mice with ovalbumin-induced food allergy].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017 May;33(5):597-600. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017. PMID: 28502295 Chinese.
-
Amelioration of ovalbumin induced allergic symptoms in Balb/c mice by potentially probiotic strains of lactobacilli.Benef Microbes. 2015;6(5):669-78. doi: 10.3920/BM2014.0141. Epub 2015 Apr 22. Benef Microbes. 2015. PMID: 25869278
-
Colonization and Gut Flora Modulation of Lactobacillus kefiranofaciens ZW3 in the Intestinal Tract of Mice.Probiotics Antimicrob Proteins. 2018 Jun;10(2):374-382. doi: 10.1007/s12602-017-9288-4. Probiotics Antimicrob Proteins. 2018. PMID: 28578494
-
Immunomodulatory and anti-allergic effects of orally administered Lactobacillus species in ovalbumin-sensitized mice.J Microbiol Biotechnol. 2013 May;23(5):724-30. doi: 10.4014/jmb.1211.11079. J Microbiol Biotechnol. 2013. PMID: 23648865
-
Oral administration of Lactobacillus plantarum JC7 alleviates OVA-induced murine food allergy through immunoregulation and restoring disordered intestinal microbiota.Eur J Nutr. 2023 Mar;62(2):685-698. doi: 10.1007/s00394-022-03016-5. Epub 2022 Oct 4. Eur J Nutr. 2023. PMID: 36194269 Free PMC article.
References
-
- Bel Imam M., Stikas C.V., Guha P., Chawes B.L., Chu D., Greenhawt M., Khaleva E., Munblit D., Nekliudov N., van de Veen W., et al. Outcomes reported in randomized controlled trials for mixed and non-IgE-mediated food allergy: Systematic review. Clin. Exp. Allergy. 2023;53:526–535. doi: 10.1111/cea.14304. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

