Role of Increasing Body Mass Index in Gut Barrier Dysfunction, Systemic Inflammation, and Metabolic Dysregulation in Obesity
- PMID: 39796506
- PMCID: PMC11723324
- DOI: 10.3390/nu17010072
Role of Increasing Body Mass Index in Gut Barrier Dysfunction, Systemic Inflammation, and Metabolic Dysregulation in Obesity
Abstract
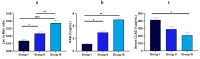
Aims: This study explores the link between body mass index (BMI), intestinal permeability, and associated changes in anthropometric and impedance parameters, lipid profiles, inflammatory markers, fecal metabolites, and gut microbiota taxa composition in participants having excessive body mass.
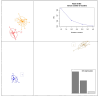
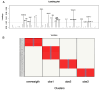
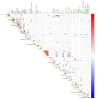
Methods: A cohort of 58 obese individuals with comparable diet, age, and height was divided into three groups based on a priori clustering analyses that fit with BMI class ranges: Group I (25-29.9), Group II (30-39.9), and Group III (>40). Anthropometric and clinical parameters were assessed, including plasma C-reactive protein and cytokine profiles as inflammation markers. Intestinal permeability was measured using a multisaccharide assay, with fecal/serum zonulin and serum claudin-5 and claudin-15 levels. Fecal microbiota composition and metabolomic profiles were analyzed using a phylogenetic microarray and GC-MS techniques.
Results: The statistical analyses of the clinical parameters were based on the full sample set, whereas a subset composed of 37 randomized patients was inspected for the GC/MS metabolite profiling of fecal specimens. An increase in potentially pro-inflammatory bacterial genera (e.g., Slackia, Dorea, Granulicatella) and a reduction in beneficial genera (e.g., Adlercreutzia, Clostridia UCG-014, Roseburia) were measured. The gas chromatography/mass spectrometry analysis of urine samples evidenced a statistically significant increase in m-cymen-8-ol, 1,3,5-Undecatriene, (E, Z) and a decreased concentration of p-cresol, carvone, p-cresol, and nonane.
Conclusions: Together, these data demonstrated how an increased BMI led to significant changes in inflammatory markers, intestinal barrier metabolites, glucose metabolism, endocrine indicators, and fecal metabolomic profiles that can indicate a different metabolite production from gut microbiota. Our findings suggest that targeting intestinal permeability may offer a therapeutic approach to prevent and manage obesity and related metabolic complications, reinforcing the link between gut barrier function and obesity.
Keywords: body mass index (BMI); fecal metabolomics; fecal microbiota; inflammatory markers; intestinal permeability; obesity; zonulin.
Conflict of interest statement
The authors declare that the research was performed without any financial relationships that could represent a possible conflict of interest.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

