Effect of Proanthocyanidins from Grape Seed Extract on Benign Prostatic Hyperplasia
- PMID: 39796507
- PMCID: PMC11723264
- DOI: 10.3390/nu17010073
Effect of Proanthocyanidins from Grape Seed Extract on Benign Prostatic Hyperplasia
Abstract
Background/objectives: Benign prostatic hyperplasia (BPH) is one of the most common chronic diseases affecting the urinary tract that occurs mainly in men over 40 years of age. Among the natural therapies, proanthocyanidins (PACs), which can treat a wide range of immune-mediated inflammatory diseases (IMIDs), have been shown to play an important role in the treatment of pathologies concerning prostate health. In this regard, the present study aimed to evaluate the different bioactivities of a grape seed extract (GSE), rich in polymeric PACs, and its version processed under alkaline conditions (ATGSE), characterized by a higher content of oligomeric PACs, in an animal model of BPH induced by subcutaneous injection of testosterone (1 mg/mouse).
Methods: These latter were divided into a control group (vehicle, olive oil), a BPH group (testosterone 1 mg/mouse), and four treatment groups treated with GSE (500 mg/kg) and ATGSE (125, 250, 500 mg/kg) by oral gavage. At the experimental endpoint (4 weeks), hematological and biochemical analyses of blood and tissues were performed.
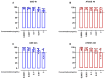
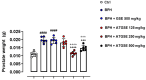
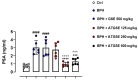
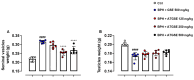
Results: Data showed that oral administration of ATGSE (250 mg/kg) was significantly more effective than GSE in reducing prostate (p ≤ 0.0001) and seminal vesicle (p ≤ 0.0001) weight. Moreover, ATGSE exhibited enhanced effectiveness in significantly reducing PSA levels (p ≤ 0.0001 vs. GSE) and the expression of key pro-inflammatory cyto-chemokines in prostate and seminal vesicles homogenates.
Conclusions: These findings pave the way for the clinical application of ATGSE as a nutraceutical and/or functional food.
Keywords: Vitis vinifera; benign prostatic hyperplasia; grape seeds; proanthocyanidins.
Conflict of interest statement
Author Elisabetta Schiano is currently employed by the research company Inventia Biotech s.r.l. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Barkin J. Benign Prostatic Hyperplasia and Lower Urinary Tract Symptoms: Evidence and Approaches for Best Case Management. Can. J. Urol. 2011;18:14–19. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

