Plasma Bacterial Metabolites in Crohn's Disease Pathogenesis and Complications
- PMID: 39796508
- PMCID: PMC11722665
- DOI: 10.3390/nu17010074
Plasma Bacterial Metabolites in Crohn's Disease Pathogenesis and Complications
Abstract
Background/objectives: Crohn's disease is known for being associated with an abnormal composition of the bacterial flora, dysbiosis and intestinal function disorders. Metabolites produced by gut microbiota play a pivotal role in the pathogenesis of CD, and the presence of unspecific extraintestinal manifestations.
Methods: The aim of this study was a determination of the level of bacterial metabolites in blood plasma in patients with Crohn's disease. CD patients (29) and healthy individuals (30) were recruited for this study. Bacterial metabolites (SCFAs and TMAO panel) were measured by a liquid chromatography-mass spectrometry system.
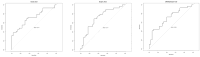
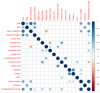
Results: A significant correlation (p-value < 0.05) between CD and bacterial metabolites was obtained for three of eight tested SCFAs; acetic acid (reduced in CD; FC 1.7; AUC = 0.714), butyric acid (increased; FC 0.68; AUC = 0.717), 2MeBA (FC 1.168; AUC = 0.702), and indoxyl (FC 0.624). The concentration of CA (FC 0.82) and choline (FC 0.78) in plasma was significantly disturbed according to the biological treatment. Choline level (FC 1.28) was also significantly disturbed in the patients treated with glucocorticoids. In total, 68.97% of Crohn's patients presented extraintestinal manifestations (EIMs) of Crohn's disease, mainly osteoarticular complications. The level of BA was statistically significantly elevated in patients with extraintestinal (FC 0.602) manifestations, while in the group of patients with osteoarticular complications, a significant difference in the level of betaine (FC 1.647) was observed.
Conclusions: The analyzed bacterial metabolites of plasma may significantly help in the diagnostic process, and in the monitoring of the disease course and treatment, in a lowly invasive way, as biomarkers after additional research on a larger group of patients.
Keywords: Crohn’s disease; SCFA; TMAO; gut microbiome; inflammatory bowel diseases; metabolites; metabolome.
Conflict of interest statement
Author Agnieszka Paziewska was employed by the company Warsaw Genomics Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

