Therapeutic Potential of Ketogenic Interventions for Autosomal-Dominant Polycystic Kidney Disease: A Systematic Review
- PMID: 39796576
- PMCID: PMC11723166
- DOI: 10.3390/nu17010145
Therapeutic Potential of Ketogenic Interventions for Autosomal-Dominant Polycystic Kidney Disease: A Systematic Review
Abstract
Background: Recent findings have highlighted that abnormal energy metabolism is a key feature of autosomal-dominant polycystic kidney disease (ADPKD). Emerging evidence suggests that nutritional ketosis could offer therapeutic benefits, including potentially slowing or even reversing disease progression. This systematic review aims to synthesise the literature on ketogenic interventions to evaluate the impact in ADPKD.
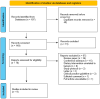
Methods: A systematic search was conducted in Medline, Embase, and Scopus using relevant Medical Subject Headings (MeSH) and keywords. Studies assessing ketogenic interventions in the management of ADPKD in both human and animal models were selected for data extraction and analysis.
Results: Three animal reports and six human studies were identified. Ketogenic diets (KD) significantly slowed polycystic kidney disease (PKD) progression in rats with improved renal function and reduced cystic areas. There was reduced renal fibrosis and cell proliferation. The supplementation of beta-hydroxybutyrate (BHB) in rats also reduced PKD progression in a dose-dependent manner. Human studies (n = 129) on KD in ADPKD reported consistent body mass index (BMI) reduction across trials, with an average weight loss of ∼4 kg. Improvements in blood pressure were also noted. Ketosis was achieved in varying degrees. Effects on kidney function (eGFR) were beneficial. Results for kidney volume were mixed but most studies were underpowered for this outcome. Lipid profiles showed increases in total cholesterol (∼1 mmol/L) and LDL cholesterol (∼0.4 mmol/L) in most studies. Safety concerns such as "keto flu" symptoms, elevated uric acid levels, and occasional kidney stones were noted. Overall feasibility and adherence to the KD were rated positively by most participants.
Conclusions: Human studies are promising; however, they have been limited by small sample sizes and short durations. Larger, long-term trials are needed to assess the efficacy, adherence, and safety of ketogenic diets in people with ADPKD.
Keywords: autosomal-dominant polycystic kidney disease; ketogenic diet; ketogenic metabolic therapy; ketone bodies; ketosis; polycystic kidney disease.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Interventions for preventing the progression of autosomal dominant polycystic kidney disease.Cochrane Database Syst Rev. 2024 Oct 2;10(10):CD010294. doi: 10.1002/14651858.CD010294.pub3. Cochrane Database Syst Rev. 2024. PMID: 39356039
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Interventions for preventing the progression of autosomal dominant polycystic kidney disease.Cochrane Database Syst Rev. 2015 Jul 14;2015(7):CD010294. doi: 10.1002/14651858.CD010294.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2024 Oct 2;10:CD010294. doi: 10.1002/14651858.CD010294.pub3. PMID: 26171904 Free PMC article. Updated.
-
Ketogenic diet and other dietary treatments for epilepsy.Cochrane Database Syst Rev. 2016 Feb 9;2:CD001903. doi: 10.1002/14651858.CD001903.pub3. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2018 Nov 07;11:CD001903. doi: 10.1002/14651858.CD001903.pub4. PMID: 26859528 Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
Cited by
-
Metabolic Reprogramming in Autosomal Dominant Polycystic Kidney Disease: Role in Cystogenesis and Novel Therapeutic Approaches.Biomedicines. 2025 Jun 30;13(7):1596. doi: 10.3390/biomedicines13071596. Biomedicines. 2025. PMID: 40722668 Free PMC article. Review.
-
Nutritional considerations for designing ketogenic dietary interventions for people with Autosomal Dominant Polycystic Kidney Disease.J Nephrol. 2025 Aug 10. doi: 10.1007/s40620-025-02378-3. Online ahead of print. J Nephrol. 2025. PMID: 40783883 Review.
References
-
- Aung T.T., Bhandari S.K., Chen Q., Malik F.T., Willey C.J., Reynolds K., Jacobsen S.J., Sim J.J. Autosomal Dominant Polycystic Kidney Disease Prevalence among a Racially Diverse United States Population, 2002 through 2018. Kidney360. 2021;2:2010–2015. doi: 10.34067/KID.0004522021. - DOI - PMC - PubMed
-
- Spithoven E.M., Kramer A., Meijer E., Orskov B., Wanner C., Caskey F., Collart F., Finne P., Fogarty D.G., Groothoff J.W., et al. Analysis of data from the ERA-EDTA Registry indicates that conventional treatments for chronic kidney disease do not reduce the need for renal replacement therapy in autosomal dominant polycystic kidney disease. Kidney Int. 2014;86:1244–1252. doi: 10.1038/ki.2014.120. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

