Dietary Influences on Gut Microbiota and Their Role in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)
- PMID: 39796579
- PMCID: PMC11722922
- DOI: 10.3390/nu17010143
Dietary Influences on Gut Microbiota and Their Role in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)
Abstract
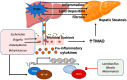
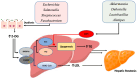
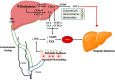
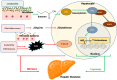
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a major contributor to liver-related morbidity, cardiovascular disease, and metabolic complications. Lifestyle interventions, including diet and exercise, are first line in treating MASLD. Dietary approaches such as the low-glycemic-index Mediterranean diet, the ketogenic diet, intermittent fasting, and high fiber diets have demonstrated potential in addressing the metabolic dysfunction underlying this condition. The development and progression of MASLD are closely associated with taxonomic shifts in gut microbial communities, a relationship well-documented in the literature. Given the importance of diet as a primary treatment for MASLD, it is important to understand how gut microbiota and their metabolic byproducts mediate favorable outcomes induced by healthy dietary patterns. Conversely, microbiota changes conferred by unhealthy dietary patterns such as the Western diet may induce dysbiosis and influence steatotic liver disease through promoting hepatic inflammation, up-regulating lipogenesis, dysregulating bile acid metabolism, increasing insulin resistance, and causing oxidative damage in hepatocytes. Although emerging evidence has identified links between diet, microbiota, and development of MASLD, significant gaps remain in understanding specific microbial roles, metabolite pathways, host interactions, and causal relationships. Therefore, this review aims to provide mechanistic insights into the role of microbiota-mediated processes through the analysis of both healthy and unhealthy dietary patterns and their contribution to MASLD pathophysiology. By better elucidating the interplay between dietary nutrients, microbiota-mediated processes, and the onset and progression of steatotic liver disease, this work aims to identify new opportunities for targeted dietary interventions to treat MASLD efficiently.
Keywords: Mediterranean diet; Western diet; gut bacteria; intermittent fasting; ketogenic diet; liver disease.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Dietary Strategies to Modulate Gut Microbiota in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD).Nutrients. 2025 Jun 1;17(11):1906. doi: 10.3390/nu17111906. Nutrients. 2025. PMID: 40507175 Free PMC article. Review.
-
Advances in the acting mechanism and treatment of gut microbiota in metabolic dysfunction-associated steatotic liver disease.Gut Microbes. 2025 Dec;17(1):2500099. doi: 10.1080/19490976.2025.2500099. Epub 2025 May 20. Gut Microbes. 2025. PMID: 40394806 Free PMC article. Review.
-
Gut microbiota in patients with metabolic, dysfunction-associated steatotic liver disease.Curr Opin Clin Nutr Metab Care. 2025 Jul 1;28(4):307-315. doi: 10.1097/MCO.0000000000001128. Epub 2025 Apr 23. Curr Opin Clin Nutr Metab Care. 2025. PMID: 40294087 Review.
-
Dietary inflammatory potential and metabolic (dysfunction)-associated steatotic liver disease and its complications: A comprehensive review.Clin Nutr ESPEN. 2025 Feb;65:162-171. doi: 10.1016/j.clnesp.2024.11.032. Epub 2024 Nov 26. Clin Nutr ESPEN. 2025. PMID: 39608495 Review.
-
Breaking the barriers: the role of gut homeostasis in Metabolic-Associated Steatotic Liver Disease (MASLD).Gut Microbes. 2024 Jan-Dec;16(1):2331460. doi: 10.1080/19490976.2024.2331460. Epub 2024 Mar 21. Gut Microbes. 2024. PMID: 38512763 Free PMC article. Review.
Cited by
-
The Influence of Physical Exercise, Ketogenic Diet, and Time-Restricted Eating on De Novo Lipogenesis: A Narrative Review.Nutrients. 2025 Feb 13;17(4):663. doi: 10.3390/nu17040663. Nutrients. 2025. PMID: 40004991 Free PMC article. Review.
-
Unraveling MASLD: The Role of Gut Microbiota, Dietary Modulation, and AI-Driven Lifestyle Interventions.Nutrients. 2025 May 4;17(9):1580. doi: 10.3390/nu17091580. Nutrients. 2025. PMID: 40362889 Free PMC article. Review.
-
Association of dietary inflammatory index on all-cause and cardiovascular mortality in U.S. adults with metabolic dysfunction associated steatotic liver disease.Front Nutr. 2025 Apr 1;12:1478165. doi: 10.3389/fnut.2025.1478165. eCollection 2025. Front Nutr. 2025. PMID: 40242166 Free PMC article.
-
Gut Microbiota and Its Metabolite Taurine-β-Muricholic Acid Contribute to Antimony- and/or Copper-Induced Liver Inflammation.Int J Mol Sci. 2025 Apr 3;26(7):3332. doi: 10.3390/ijms26073332. Int J Mol Sci. 2025. PMID: 40244173 Free PMC article.
-
Association of dietary inflammatory index with liver fibrosis and fatty liver index in a population with metabolic dysfunction-associated steatotic liver disease: A cross-sectional study.Front Nutr. 2025 Jun 24;12:1594192. doi: 10.3389/fnut.2025.1594192. eCollection 2025. Front Nutr. 2025. PMID: 40630170 Free PMC article.
References
-
- Kanwal F., Neuschwander-Tetri B.A., Loomba R., Rinella M.E. Metabolic dysfunction-associated steatotic liver disease: Update and impact of new nomenclature on the American Association for the Study of Liver Diseases practice guidance on nonalcoholic fatty liver disease. Hepatology. 2024;79:1212–1219. doi: 10.1097/HEP.0000000000000670. - DOI - PubMed
-
- Younossi Z.M., Alqahtani S.A., Alswat K., Yilmaz Y., Keklikkiran C., Funuyet-Salas J., Romero-Gómez M., Fan J.G., Zheng M.H., El-Kassas M., et al. Global survey of stigma among physicians and patients with nonalcoholic fatty liver disease. J. Hepatol. 2024;80:419–430. doi: 10.1016/j.jhep.2023.11.004. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

