Cell Membrane Fatty Acids and PIPs Modulate the Etiology of Pancreatic Cancer by Regulating AKT
- PMID: 39796583
- PMCID: PMC11722924
- DOI: 10.3390/nu17010150
Cell Membrane Fatty Acids and PIPs Modulate the Etiology of Pancreatic Cancer by Regulating AKT
Abstract
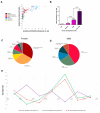
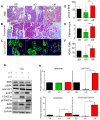
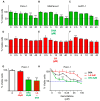
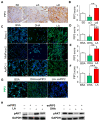
Background: Pancreatic ductal adenocarcinoma (PDAC) is one of the worst solid malignancies in regard to outcomes and metabolic dysfunction leading to cachexia. It is alarming that PDAC incidence rates continue to increase and warrant the need for innovative approaches to combat this disease. Due to its relatively slow progression (10-20 years), prevention strategies represent an effective means to improve outcomes. One of the risk factors for many cancers and for pancreatic cancer in particular is diet. Hence, our objective is to understand how a diet rich in ω3 and ω6 polyunsaturated fatty acids affects the progression of this disease. Methods: We investigated polyunsaturated fatty acid (PUFA) effects on disease progression employing both in vitro (PDAC cell lines) and in vivo (EL-Kras and KC mice) approaches. Also, we gathered data from the National Health and Nutrition Examination Survey (NHANES) and the National Cancer Institute (NCI) from 1999 to 2017 for a retrospective observational study. Results: The consumption of PUFAs in a patient population correlates with increased PDAC incidence, particularly when the ω3 intake increases to a lesser extent than ω6. Our data demonstrate dietary PUFAs can be incorporated into plasma membrane lipids affecting PI3K/AKT signaling and support the emergence of membrane-targeted therapies. Moreover, we show that the phospholipid composition of a lipid nanoparticle (LNP) can impact the cell membrane integrity and, ultimately, cell viability after administration of these LNPs. Conclusions: Cancer prevention is impactful particularly for those with very poor prognosis, including pancreatic cancer. Our results point to the importance of dietary intervention in this disease when detected early and the potential to improve the antiproliferative effect of drug efficacy when combined with these regimens in later stages of pancreatic cancer.
Keywords: PDAC; PI3K/AKT; PIP2 (phosphatidylinositol 4,5-bisphosphate); PIP3 (phosphatidylinositol (3,4,5)-trisphosphate); diet; polyunsaturated fatty acids; prevention.
Conflict of interest statement
All authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest or nonfinancial interest in the subject matter or materials discussed in this manuscript.
Figures


References
-
- Abdilleh K., Khalid O., Ladnier D., Wan W., Seepo S., Rupp G., Corelj V., Worman Z.F., Sain D., DiGiovanna J., et al. Pancreatic Cancer Action Network’s SPARK: A Cloud-Based Patient Health Data and Analytics Platform for Pancreatic Cancer. JCO Clin. Cancer Inform. 2024;8:e2300119. doi: 10.1200/CCI.23.00119. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

