Growth, Safety and Tolerance in Infants Fed Rice Protein Hydrolysate Formula: The GRITO Randomised Controlled Trial
- PMID: 39796596
- PMCID: PMC11722687
- DOI: 10.3390/nu17010162
Growth, Safety and Tolerance in Infants Fed Rice Protein Hydrolysate Formula: The GRITO Randomised Controlled Trial
Abstract
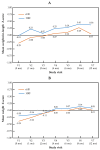
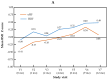
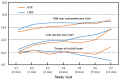
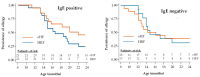
Background: Hydrolysed rice formula (HRF) is tolerated by >90% of children with cow's milk protein allergy (CMPA). However, concerns have been raised about potential suboptimal growth in infants fed HRF compared to those fed an extensively hydrolysed milk protein formula (eHF). Aims: To compare growth, safety and tolerance acquisition in infants with CMPA when fed HRF versus eHF. Methods: A multicentre prospective, randomised, double-blind, placebo-controlled food challenge trial was conducted with infants with CMPA. The infants received either HRF or eHF over a 12-month follow-up period. The primary outcome measure was the change from baseline over the study period in weight-for-length expressed as a Z-score. The secondary outcomes were other anthropometric measurements, tolerability and adverse events (AEs). Results: In total, 105 children were enrolled. The weight-for-length measurements were -0.01 (HRF) and -0.29 (eHF) at baseline and 0.29 and 0.05, respectively, at the last visit, with no significant between-group difference (p = 0.28; mixed-effects model). The Z-scores for other anthropometric variables indicated normal growth, with no significant between-group differences. In total, 29 potentially product-related AEs were reported (12 in the HRF group and 17 in the eHF group). A trend was observed toward a faster acquisition of tolerance in the HRF group (median age: 20.4 months) compared to the eHF group (16.3 months), but this was not statistically significant (p = 0.18). Conclusions: HRF demonstrated appropriate growth, acquisition of tolerance and a good safety profile in infants with CMPA, with no significant differences versus eHF. HRF could be considered as an appropriate option in the management of CMPA.
Keywords: arsenic; children; cow’s milk protein allergy; hydrolysed rice-based formula; tolerance.
Conflict of interest statement
A.L.: none to declare. A.N.-G.: none to declare. M.N.-C: none to declare. B.E.-J.: has received honoraria for lectures from Alter, Mead Johnson, Ferrer, Nestle, Nutricia/Danone, Hero and Abbott and has participated in advisory boards for Mead Johnson, Nutricia/Danone, Ferrer and Abbott. Á.M.: none to declare. H.S.: is an employee of Laboratoires Modilac (France) which co-funded the study. D.S.: none to declare. N.K: study honoraria and presentation with conferences expenses from Laboratoires Modilac France, Laboratoires NIH, France, Laboratoires Nutricia, France, and Laboratoires Dr Falk, France. J.D.O.: was an employee of Laboratorios Ordesa (Spain), which co-funded the study. R.d.C.-S.: is an employee of Ordesa Laboratory (Spain), which co-funded the study. V.M.N.-L.: has received honoraria for lectures from Mead Johnson, Ferrer, Nestle, Nutricia/Danone, Hero and Abbott.
Figures



References
-
- Vandenplas Y., Broekaert I., Domellöf M., Indrio F., Lapillonne A., Pienar C., Ribes-Koninckx C., Shamir R., Szajewska H., Thapar N., et al. An ESPGHAN position paper on the diagnosis, management and prevention of cow’s milk allergy. J. Pediatr. Gastroenterol. Nutr. 2023;78:386–413. doi: 10.1097/MPG.0000000000003897. - DOI - PubMed
-
- Agostoni C., Fiocchi A., Riva E., Terracciano L., Sarratud T., Martelli A., Lodi F., D’Auria E., Zuccotti G., Giovannini M. Growth of infants with IgE-mediated cow’s milk allergy fed different formulas in the complementary feeding period. Pediatr. Allergy Immunol. 2007;18:599–606. doi: 10.1111/j.1399-3038.2007.00566.x. - DOI - PubMed
-
- Klemola T., Vanto T., Juntunen-Backman K., Kalimo K., Korpela R., Varjonen E. Allergy to soy formula and to extensively hydrolyzed whey formula in infants with cow’s milk allergy: A prospective, randomized study with a follow-up to the age of 2 years. J. Pediatr. 2002;140:219–224. doi: 10.1067/mpd.2002.121935. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

