Implantable Passive Sensors for Biomedical Applications
- PMID: 39796923
- PMCID: PMC11723123
- DOI: 10.3390/s25010133
Implantable Passive Sensors for Biomedical Applications
Abstract
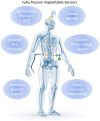
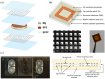
In recent years, implantable sensors have been extensively researched since they allow localized sensing at an area of interest (e.g., within the vicinity of a surgical site or other implant). They allow unobtrusive and potentially continuous sensing, enabling greater specificity, early warning capabilities, and thus timely clinical intervention. Wireless remote interrogation of the implanted sensor is typically achieved using radio frequency (RF), inductive coupling or ultrasound through an external device. Two categories of implantable sensors are available, namely active and passive. Active sensors offer greater capabilities, such as on-node signal and data processing, multiplexing and multimodal sensing, while also allowing lower detection limits, the possibility to encode patient sensitive information and bidirectional communication. However, they require an energy source to operate. Battery implantation, and maintenance, remains a very important constraint in many implantable applications even though energy can be provided wirelessly through the external device, in some cases. On the other hand, passive sensors offer the possibility of detection without the need for a local energy source or active electronics. They also offer significant advantages in the areas of system complexity, cost and size. In this review, implantable passive sensor technologies will be discussed along with their communication and readout schemes. Materials, detection strategies and clinical applications of passive sensors will be described. Advantages over active sensor technologies will be highlighted, as well as critical aspects related to packaging and biocompatibility.
Keywords: capacitive diaphragm; galvanic coupling; implantable sensors; inductive coupling; passive sensors; radiative coupling; ultrasonic coupling.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Sazonov E. Wearable Sensors: Fundamentals, Implementation and Applications. 2nd ed. Academic Press; Cambridge, MA, USA: 2020.
-
- Yang G.-Z., editor. Implantable Sensors and Systems: From Theory to Practice. Springer; Berlin, Germany: 2018.
-
- Beck H., Boden W.E., Patibandla S., Kireyev D., Gupta V., Campagna F., Cain M.E., Marine J.E. 50th Anniversary of the First Successful Permanent Pacemaker Implantation in the United States: Historical Review and Future Directions. Am. J. Cardiol. 2010;106:810–818. doi: 10.1016/j.amjcard.2010.04.043. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

