Efficacy and Safety of a Mixture of Microencapsulated Sodium Butyrate, Probiotics, and Short Chain Fructooligosaccharides in Patients with Irritable Bowel Syndrome-A Randomized, Double-Blind, Placebo-Controlled Study
- PMID: 39797089
- PMCID: PMC11720862
- DOI: 10.3390/jcm14010006
Efficacy and Safety of a Mixture of Microencapsulated Sodium Butyrate, Probiotics, and Short Chain Fructooligosaccharides in Patients with Irritable Bowel Syndrome-A Randomized, Double-Blind, Placebo-Controlled Study
Abstract
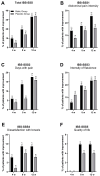
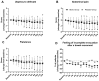
Objective: Biotics are increasingly being used in the treatment of irritable bowel syndrome (IBS). This study aimed to assess the efficacy and safety of a mixture of microencapsulated sodium butyrate, probiotics (Lactocaseibacillus rhamnosus DSM 26357, Lactobacillus acidophilus DSM 32418, Bifidobacterium longum DSM 32946, Bifidobacterium bifidum DSM 32403, and Bifidobacterium lactis DSM 32269), and short-chain fructooligosaccharides (scFOSs) in IBS patients. Methods: This was a randomized, double-blind, placebo-controlled trial involving 120 adult participants with IBS. The primary outcome of the 12-week intervention was the improvement in IBS symptoms and quality of life (QOL), assessed with the use of IBS-Adequate Relief (IBS-AR), IBS-Global Improvement Scale (IBS-GIS), IBS-Symptom Severity Score (IBS-SSS), and IBS-QOL. Secondary outcomes were the number and type of stools (assessed via the Bristol Stool Form scale), patient-recorded symptoms, anthropometric parameters, and levels of selected inflammatory cytokines. Results: As early as at 4 weeks, there was a higher percentage of patients in the biotic group reporting adequate relief of symptoms (based on IBS-AR) than in the placebo group (64.7% vs. 42.0%, respectively, p = 0.023). At 12 weeks, fewer patients in the biotic group reported a 'worsening of symptoms' (based on IBS-GIS) than in the placebo group (5.9% vs. 16.0% respectively, p = 0.015). There were no significant differences between groups in IBS-QOL or IBS-SSS or any of the secondary outcome measures except the patient-recorded 'urgency to defecate' (p = 0.015) at week 12, which was significantly lower in the biotic group. The intervention was safe and well tolerated. Conclusions: A biotic mixture consisting of microencapsulated butyrate, probiotics, and small amounts of scFOSs is safe and effective in improving gastrointestinal symptoms in patients with IBS.
Keywords: irritable bowel syndrome; microencapsulated sodium butyrate; prebiotics; probiotics; synbiotics.
Conflict of interest statement
A.G., M.R., E.W.-K., M.P., M.S., A.K., C.C., J.B., A.S.-W., M.W.-J., and J.B.B. received a grant financed by DSM-Firmenich (Kaiseraugst, Switzerland). B.C. is a scientific chief of Nordic Biotic Ltd. B.C. has conducted projects funded by Danone, Biomed S.A., and Nordic Biotic Ltd. She has also given lectures sponsored by Apotex, Nutricia, Danone, Mead Johnson, Bayer, Aurovitas, Polpharma, and Biomed. S.A., R.E.S., and N.O. are employees of DSM-Firmenich. The other authors declare no conflicts of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data as well as in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Porcari S., Ingrosso M.R., Maida M., Eusebi L.H., Black C., Gasbarrini A., Cammarota G., Ford A.C., Ianiro G. Prevalence of irritable bowel syndrome and functional dyspepsia after acute gastroenteritis: Systematic review and meta-analysis. Gut. 2024;73:1431–1440. doi: 10.1136/gutjnl-2023-331835. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

