Comparative effectiveness and safety of single inhaler triple therapies for chronic obstructive pulmonary disease: new user cohort study
- PMID: 39797646
- PMCID: PMC11684032
- DOI: 10.1136/bmj-2024-080409
Comparative effectiveness and safety of single inhaler triple therapies for chronic obstructive pulmonary disease: new user cohort study
Abstract
Objective: To compare the effectiveness and safety of budesonide-glycopyrrolate-formoterol, a twice daily metered dose inhaler, and fluticasone-umeclidinium-vilanterol, a once daily dry powder inhaler, in patients with chronic obstructive pulmonary disease (COPD) treated in routine clinical practice.
Design: New user cohort study.
Setting: Longitudinal commercial US claims data.
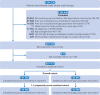
Participants: New initiators of budesonide-glycopyrrolate-formoterol or fluticasone-umeclidinium-vilanterol between 1 January 2021 and 30 September 2023 who had a diagnosis of COPD and were aged 40 years or older.
Main outcome measures: In this 1:1 propensity score matched study, the main outcome measures were first moderate or severe COPD exacerbation (effectiveness) and first admission to hospital with pneumonia (safety) while on treatment. Potential confounders were measured in the 365 days before cohort entry and included in propensity scores. Hazard ratios and 95% confidence intervals (CIs) were estimated using a Cox proportional hazards regression model.
Results: The study cohort included 20 388 propensity score matched pairs of new users initiating single inhaler triple therapy. Patients who received budesonide-glycopyrrolate-formoterol had a 9% higher incidence of first moderate or severe COPD exacerbation (hazard ratio 1.09 (95% CI 1.04 to 1.14); number needed to harm 38) compared with patients receiving fluticasone-umeclidinium-vilanterol and an identical incidence of first admission to hospital with pneumonia (1.00 (0.91 to 1.10)). The hazard of first moderate COPD exacerbation was 7% higher (1.07 (1.02 to 1.12); number needed to harm 54) and the hazard of first severe COPD exacerbation 29% higher (1.29 (1.12 to 1.48); number needed to harm 97) among those receiving budesonide-glycopyrrolate-formoterol compared to fluticasone-umeclidinium-vilanterol. Prespecified sensitivity analyses yielded similar findings to the primary analysis.
Conclusions: Budesonide-glycopyrrolate-formoterol was not associated with improved clinical outcomes compared with fluticasone-umeclidinium-vilanterol. Given the added climate impact of metered dose inhalers, health systems seeking to decrease use of these products may consider steps to promote further prescribing of fluticasone-umeclidinium-vilanterol compared with budesonide-glycopyrrolate-formoterol in people with COPD.
Study registration: Center for Open Science Real World Evidence Registry (https://osf.io/6gdyp/).
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests:All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: funding from the National Heart, Lung, and Blood Institute; outside the submitted work, WBF serves as a consultant for Alosa Health and as an expert witness in litigation against inhaler manufacturers. He previously served as a consultant for Aetion. SS attended advisory committee meetings for Atara, Novartis and Panalgo, and received speaking fees from Covis Pharma and Novartis. SS is participating in investigator-initiated grants to the Brigham and Women’s Hospital from Boehringer Ingelheim, Takeda, and UCB unrelated to the topic of this study. He owns equity in Aetion Inc, a software manufacturer. He is an advisor to Temedica GmbH, a patient-oriented data generation company. His interests were declared, reviewed, and approved by the Brigham and Women’s Hospital in accordance with their institutional compliance policies. SVW has been a consultant for Veracity Healthcare Analytics, Exponent Inc, and MITRE a federally funded research and development center for the Centers for Medicare and Medicaid for unrelated work.
Figures


References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for Prevention, Diagnosis and Management of COPD: 2024 Report. Available from: https://goldcopd.org/2024-gold-report/. Accessed February 15, 2024.
-
- Bell JPRA, Khezrian M, Kocks JW, Usamani OS. An assessment of pressurized metered-dose inhaler use in countries in europe and the rest of the world. Am J Respir Crit Care Med 2023;207:A6315.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
